Renewable Alternative: Cashew Nutshell Liquid-Based Isocyanate Blocking Agent
A high-purity cardanol derived from renewable cashew nutshell liquid offers chemical features that can be successfully used in epoxy and polyurethane adhesives and sealants.






Cashew nutshell liquid (CNSL) is a non-edible, bio-based material commonly used as a building block for various resins, hardeners, and modifiers in the adhesives, coatings, and composites industries. A high-purity cardanol, 3-pentadeca-dienyl-phenol, derived from the bio-oil CNSL has been employed to replace nonyl phenol (NP) in various applications, including isocyanate blocking. Cardanol has shown many benefits (e.g., low viscosity, lower deblocking temperature, environmentally friendly) over NP and phenols as an isocyanate blocking agent. However, high deblocking temperature has limited the use of cardanol in some applications.
One of the most known deblocking pathways involves exposure to elevated temperatures (150-200°C). However, not all the substrates (e.g., plastics) and applications can accommodate such high temperatures for deblocking. Therefore, the possibility to optimize the deblocking conditions can be a valuable tool to further expand the applicability of cardanol as a label-friendly polyurethane prepolymer blocking agent.
In this study, we will present different approaches to control cardanol deblocking conditions. Key factors for controlling deblocking conditions, including catalysts, deblocking agents (amines, polyols), and solvents, have been investigated. Blocked isocyanate and its prepolymers are used in adhesives and coatings to achieve controlled curing at desirable cure conditions, enable stable 1K epoxy and polyurethane formulations, and provide improved adhesion properties and flexibility.
Materials and Methods
A high-purity cardanol was selected as a blocking agent for isocyanates and lab-prepared prepolymers. Prepolymers were prepared starting from commercially available diols (polyester, polyether) according to the following procedure: to a weighed amount of isocyanate (NCO) pre-heated at 50°C and stirred under nitrogen atmosphere, the selected diol is added dropwise, keeping the mixture temperature around 65°C. Once the addition is completed, the system is stirred under nitrogen atmosphere at 65-70°C, monitoring prepolymer formation by NCO titration (ASTM D2572). When the desired NCO content is obtained, the prepolymer is then blocked by adding cardanol, keeping the reaction temperature around 65-70°C under nitrogen flush and monitoring NCO disappearance by titration.
Deblocking temperatures were determined by differential scanning calorimetry (DSC).1 Epoxy-polyurethane hybrid adhesives composed of liquid epoxy, phenalkamine, and 30% of blocked isocyanate prepolymer were cured at room temperature for lap shear, peel strength, and glass transition temperature (Tg).
Results and Discussion
Cardanol as Isocyanate Blocking Agent and Optimization of Deblocking Temperature
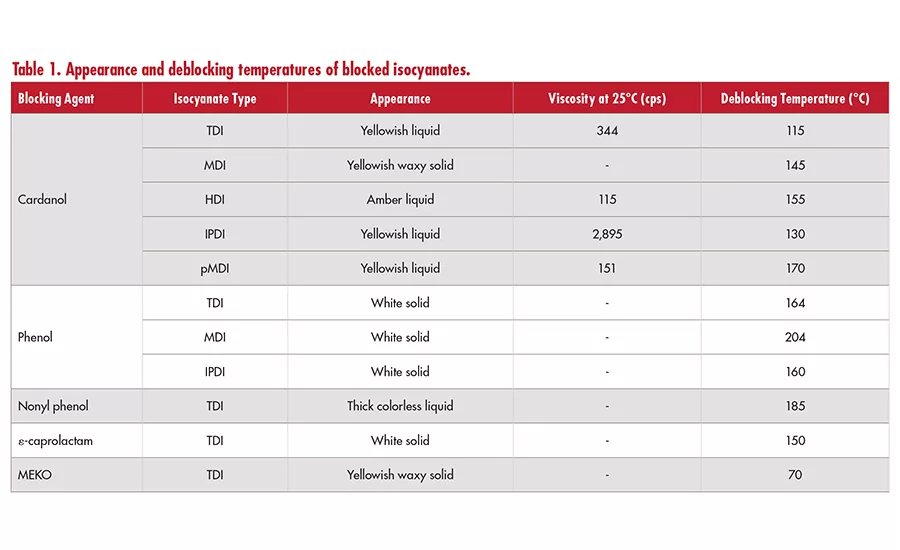
Table 1 (cardanol-blocked monomeric isocyanate) and Table 2 (cardanol-blocked polypropylene glycol prepolymer) show the benefits of using cardanol over NP or phenols, including lower viscosity, lower deblocking temperature, and faster deblocking time. However, the deblocking temperatures of cardanol can be characterized as relatively high to be used in thermosensitive materials like plastics and applications where sufficiently fast cure profiles are required.
Observed deblocking temperatures of cardanol-blocked systems were still higher than that of methylethylketoxime (MEKO) systems. Thus, it is necessary to investigate solutions to make label-friendly bio-based cardanol blocking agent to be a suitable replacement for MEKO. In this paper, the impact of catalysts, chain extenders with different nucleophilicity (e.g., polyol and amines), or their combinations have been investigated.
Properly selected catalysts, including tertiary amines, alkanolamines, and metals,2 at the use level of 0.8 wt% were evaluated in the cardanol-blocked polypropylene glycol (PPG)-based prepolymers. Deblocking temperatures from various isocyanates in a prepolymer system are displayed in Table 3.
Triethylenediamine (TEDA) in dipropyleneglycol and potassium-octoate in diethylene glycol showed the highest effect/deblocking efficiency within the series. Other options are still suitable but show a slightly lower contribution based on the type of isocyanates. The evident effect of metal catalysts (e.g., potassium octoate or bismuth carboxylate) on deblocking temperatures can be explained with their capability to generate very nucleophilic metal alkoxylates in the presence of polyols and polar solvents (as DEG, in the case of potassium octoate glycolic solution).
The study was then further extended to slightly more complex systems, evaluating the use of chain extenders (as such or in combination with catalysts) characterized by different nucleophilicity (polyols and amines). When a propoxylated sorbitol-based polyol (OH 300 mg KOH/g) was used as a potential chain extender (at a stoichiometric level with respect to the prepolymer’s equivalent weight), a reduction in deblocking temperature was observed. This became even more significant when catalysts (0.8% w/w with respect to overall substrate amount) were included in the blend (see Table 4).
As evident from the results in Tables 3 and 4, amine- and metal-type catalysts diluted with glycols showed the overall best effect in reducing the deblocking temperatures of blocked prepolymers. However, their high nucleophilicity could potentially limit their applicability in 1K systems due to the reduced shelf life of the blocked prepolymers. The investigation of alternative catalysts for balancing low deblocking temperature and increased shelf life is an ongoing effort.
Having confirmed the possibility of extending cardanol-blocked prepolymers by polyols (also reducing deblocking temperature), as well as the applicability of proper catalysts to further reduce the reaction temperature, additional experiments were conducted to evaluate the efficiency of amines as chain extenders/curing agents characterized by higher nucleophilicity (see Table 5). The same four reference model blocked prepolymers were blended with amines at a stoichiometric level using the amine’s amine hydrogen equivalent weight (AHEW) with respect to the prepolymer’s equivalent weight.
Among the three amines tested as chain extenders (isophoronediame, polyetheramine, and CNSL-phenalkamine), the amine characterized by the lowest molecular weight shows the highest effect on deblocking temperature. This is probably due to its lower steric hindrance and the subsequent easier access to reactive groups. The addition of catalysts in amine-type chain extenders can be a possible way to further reduce their deblocking temperatures.
All these results confirm the effective applicability of cardanol as a versatile NCO-blocking agent suitable for applications ranging from adhesives to coatings in outdoor applications and epoxy-PU hybrid matrices. Depending on the specific type of system (1K or 2K), catalysts or chain extenders can be blended into cardanol-blocked isocyanate-derived adducts, and their levels can be properly adjusted to maintain good balance between reactivity and formulation stability over time.
Adhesion Performance of Cardanol-Blocked Prepolymers in 2K Epoxy Formulations
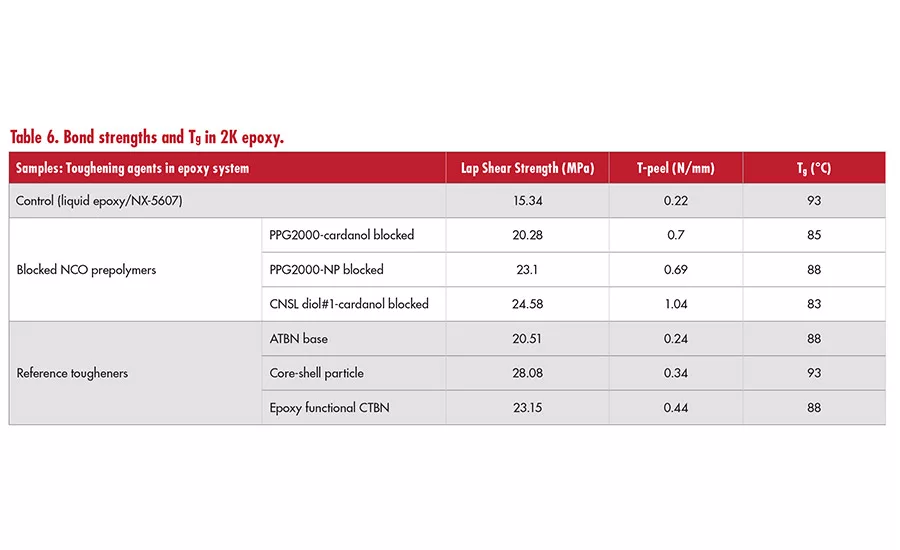
Blocked isocyanates have allowed formulators to use isocyanates in epoxy systems without stability concerns.3 As shown in Table 6, PPG-based prepolymers blocked with either cardanol or NP and one special prepolymer based on CNSL diol#1 blocked with cardanol were prepared. The prepolymers were studied in 2K epoxy systems in a blend with liquid epoxy at 30% use level and cured with a phenalkamine at room temperature.
In this study, the use of blocked prepolymer in epoxy systems provided many benefits, including increased bond strength (lap shear and T-peel) and improved flexibility, while the lowering of Tg was minimized. Moreover, it is also common to use tougheners such as ATBN or modified CTBN and core shell particles in epoxy systems to improve bond strength. Our study demonstrated that blocked prepolymer systems can offer slightly better T-peel strength than those commonly used epoxy tougheners. A similar increase in bond strengths, but with higher reduction in Tg, was observed in a 1K epoxy system (see Table 7). Combined studies suggest that PU-epoxy hybrid structures created in-situ by incorporating a blocked prepolymer deliver excellent bond strengths and a high Tg.
Conclusions
A high-purity cardanol derived from renewable cashew nutshell liquid offers chemical features that can be successfully used in epoxy and polyurethane adhesives and sealants. Cardanol has been used as an isocyanate blocking agent as an alternative to petro-based phenol and nonyl phenol due to benefits that include lower deblocking temperature, lower viscosity, and favorable labeling.
By properly selecting suitable conditions (e.g., the use of catalysts, chain extenders, or their combinations), cardanol deblocking temperatures can be significantly lowered and easily tuned. The advantages of blocked isocyanates/prepolymers in adhesives and sealants include increased bond strength and flexibility, which can help manage thermal shock in harsh conditions.
For more information, contact the lead author at (609) 436-0902 or ykim@cardolite.com, or visit www.cardolite.com.
Authors’ acknowledgements: This study was carried out by the Research and Development and Technical Service teams at Cardolite Corp. and AEP Italy.
References
- Rolph, M.S.; Markowska, A.L.J.; Warriner, C.N.; O’Reilly, R.K., Polym. Chem., 7, 7351-7364, 2016.
- a) Duncan, J.S.; Elmer, O.C. US3668186; b) Mohanty, S.; Krishnamurti, N., Eur. Polym. J., 34, 77-83, 1998; c) Truc-Chi, Huynh-Tran; Shiow, C.L., EP0261515; d) Hannah, S.L.; Williams, M.R.; Greenlee, T.W., US4798879.
- a) Schmalstieg, L.; Rettig, R.; König, E., US5510432; b) Yan, L.; Zhang, Y.; Zhou, W., WO2014067096.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!






