Corona Surface Treatments and Bonding with Silicone Adhesives

Adhesives and fastening technologies are some of the fastest growing products in the business-to-business marketplace. As technology changes rapidly, so does the need for new adhesives. Design trends like miniaturization and uses of devices in harsh environments force engineers to evaluate new materials and configurations.
Silicones used in adhesive and sealant applications can provide good bonding to substrates without the use of primers or surface modification techniques, such as corona, flame and plasma treatments; but some situations require the use of these additional components and/or processing steps to significantly boost adhesion to substrate surfaces.
Adhesion
Bonding any type of silicone requires an understanding of the mechanisms necessary for a good bond: surface energy and chemical reactivity. Surface energy is the thermodynamic effect related to a material’s intramolecular forces and is a determining factor in how an adhesive spreads across a surface. It is commonly accepted that the substrate’s surface energy must exceed that of the adhesive for adequate contact.1 Intuitively, the better an adhesive spreads, the more intimate the contact between molecules, thus allowing more reactive groups to interact or bond. In aggregate, the interactions between the adhesive and substrate make a stronger bond.Low-surface-energy materials, such as some plastics, do not allow adhesives to spread out, which means they are generally poor candidates for bonding. It is possible to raise the surface energy of some plastics through UV radiation, plasma and corona discharge, or by flame or acid treatments. The presence of oxygen containing species, such as OH groups, on the surface of plastics provides reactive sites for silicone adhesive and primer systems.
In addition to adequate surface wetting, a second critical factor is how the adhesive forms a mechanical or chemical bond to the substrate surface. Adhesion can be achieved through a few mechanisms, but, for our purposes, we will focus on chemical adhesion. Chemical adhesion is defined as the chemical bonding of two substrates by covalent bonding, hydrogen bonding or other Van der Waals forces. Substrates with reactive groups available for bonding - like OH groups on glass and oxide layers on metals like aluminum - make this chemical bond easier to achieve. Substrates with inert surfaces, such as graphite and Polytetrafluoroethylene (PTFE), can make adhesion difficult.
Corona Treatment
Corona treatment is the modification of a substrate’s surface energy and bonding ability through the excitation of molecules due to a corona or electrical discharge. When atmospheric air is exposed to different voltage potentials, electrical discharge can develop, which can then trigger a collision of neutral molecules, causing the electrically loaded molecules to form a cloud of ionized air. The electrons generated in the corona discharge impact on the treatment surface with energies two to three times what is needed to break molecular bonds on certain plastic (non-metallic) surfaces. The resulting free radicals react rapidly with the oxidating molecules to form an oxidation layer on the substrate. These oxides increase surface energy, promote better wetting, and can deposit reactive polar groups, such as hydroxyl, carbonyl, and amide groups, onto the surface.2 Depending on the level of excitation and the stability of the electrons within their excited state, the material will retain this excited energy level for varying lengths of time.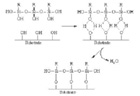
Figure
1. Primers form a compatible interface between the incompatible substrates and
promote adhesion.
Silicone Primers and Adhesives
PrimersSilane primers are used to promote adhesion between a surface and an adhesive. Although designed for use with silicone adhesives, they can also be used with other adhesives, such as epoxies or acrylates. Primers usually consist of one or more reactive silanes, a condensation catalyst and some type of solvent carrier. The reactive silanes typically have two reactive groups: one compatible with the substrate, and the other with the adhesive. Some reactive groups may be hydrophilic like a silanol (Si-OH) group or hydrophobic like a 1-octenyl group. These different groups form a compatible interface between the incompatible substrate and adhesive, promoting adhesion (see Figure 1). The reactive silanes are usually moisture-sensitive alkoxy silanes and, in the presence of atmospheric moisture and a condensation catalyst, form the priming surface. The silanes and the condensation catalysts form a very thin polymeric film on the substrate’s surface. Theoretically, the best primer film is a mono-molecular layer, with the compatible groups facing the substrate and the organic groups facing the organic silicone adhesive’s surface. In reality, these monolayers don’t exist, but compatible bi- or tri-layers do. This illustrates the importance of thin primer films and the necessity of solvent carriers in the primer formulation. Thick, overly primed surfaces tend to build chalky primer films that can be points of adhesive failure.
Figure
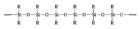
2. The chemical structure of a silicone adhesive polymer.
Silicone adhesives typically comprise a polysiloxane polymer (see Figure 2), a reinforcing filler (such as silica), a crosslinking polymer or compound, and a catalyst.
Polysiloxanes can be formulated to provide a variety of excellent elastomeric properties such as temperature stability (-115°C to 260°C), fuel resistance, optical clarity (with refractive indexes as high as 1.60), low shrinkage (2-%), and low shear stress.

Figure 3. Addition-cure systems involve the addition of a Si-H functional crosslinker to the vinyl functional polymer, forming an ethylene bridge crosslink.
Cure Systems
Another variability in silicone adhesive systems is the method of cure, with each cure system having distinct advantages and disadvantages for processing. Typically, silicone adhesives are formulated with one of the following three cure systems: condensation cure (acetoxy), oxime systems and addition cure. The study conducted involved the use of an addition-cure silicone adhesive. The key formulation components and the reaction mechanism are described below.Addition Cure
Addition-cured silicone adhesives are commonly referred to as platinum-catalyzed adhesives and are generally two-part systems, with each part containing different functional components.
These two-component systems can be formulated in various ratios, with the most common ratios being 10:1 and 1:1. Generally, the Part A component contains vinyl-functional silicones and the platinum catalyst, whereas Part B contains a vinyl-functional polymer, hydrogen-functional crosslinker and cure inhibitor. Cure inhibitors are additives used to adjust the cure rate of the system.
The cure chemistry involves the direct addition of a Si-H functional crosslinker to the vinyl functional polymer, forming an ethylene bridge crosslink (see Figure 3).
The vulcanization of addition-cured silicone elastomers can be heat accelerated. Depending on the specific product, addition-cured elastomers can be fully cured at temperatures and times ranging from 10 minutes at 116°C to 2 minutes at 150°C. Cure conditions vary with product mass (a thin section of adhesive will cure much quicker than a thick section, as the appropriate cure temperature will take longer to achieve in the core of the silicone sample). Special care must be taken to eliminate the presence of contaminants that might negatively impact the catalyst.
Substrates
Eight substrates were evaluated for the study; they were chosen based on industrial use and low surface energy. The substrates evaluated were polyurethane (PU), polycarbonate (PC), polypropylene (PP), polymethylmethacrylate (PMMA), polyetheretherketone (PEEK), polyethylene terephthalate (PET) and a GFK epoxy. Panels were prepared for each material and measured 100 x 25 x 2 mm, except for PEEK, which measured 0.63 mm in thickness. The substrates were cleaned with isopropyl alcohol prior to corona treatment.
Evaluation of Substrate Surface Energy
The evaluation of surface energy was carried out before and after each substrate was exposed to corona treatment. The surface tension of each substrate was measured visually by applying a stripe of ink of known surface energy onto the substrate’s surface. If the liquid briefly “wet out” the surface of the substrate before breaking into droplets, the authors determined the liquid and substrate had substantially the same surface energy. Ten inks were used for this study, covering a range of surface tension of 32 to 56 dynes/cm. The same procedure was performed 10 minutes and 72 hours after corona treatment, and the results are found in Table 1 (in dyne/cm).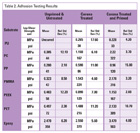
Adhesion Testing
Testing ConfigurationsThe adhesion testing used to evaluate the bond strengths in this study was based on the ASTM method of determining tensile lap shear strengths of rigid-to-rigid, bonded assemblies.3 Each test specimen was prepared by masking an area on the tip of one panel with adhesive tape of known thickness and placing another panel on top and in contact with the adhesive. The size of the overlap was 25x12.5mm, and the thickness approximately 0.4mm. Primed substrates were prepared using NuSil Technology’s MED1-161 primer in accordance with the product use instructions. The study cured the adhesive per the product instructions (NuSil Technology MED1-4013, 24 hours at room temperature). Six texts were performed for each substrate under three conditions: no treatment or primer, corona treatment only, and corona treatment and primer. The highest and lowest values were disregarded and the reported result represented the mean of the remaining four measurements (see Table 2 and Figure 4).
The equipment used to test for lap shear value was an MTS Model Alliance RT/5 with MTS data acquisition and 5kN load cell installed.
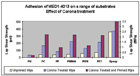
Figure
4. Adhesion testing results.
Conclusion
The study first evaluated the effect of corona treatment on a range of substrate surfaces and the effect of time passage on that treatment. It was observed that corona treatment positively raised the surface energy of all of the substrates in the study. It appears that the effects of this treatment did not change substantially over a 72-hour period. This result may have a positive impact on processing because the assembly of a device would not require an immediate use of the treated part. Future studies will evaluate tensile lap shear strength of silicone adhesives in prolonged periods after the treatment is applied.The second part of the study examined tensile lap shear strength. The results of the test are based on the “failure” of the bond. The mechanisms of failure in adhesively bonded joints are usually substrate failure, adhesive failure or cohesive failure. Substrate failure is the fracture or internal failure within the substrate, indicating that the bond is stronger than the substrate. Adhesive failure is the interfacial failure between the adhesive and the substrate. Cohesive failure is the internal failure of the adhesive itself. This indicates that the strength of the bonded materials is greater than the strength of the adhesive’s physical properties. Usually, the failure of joints is neither completely cohesive nor completely adhesive. Measurement of the success of a particular joint is based on the relative percentage of cohesive failure to adhesive failure.
In this study, adhesive failure was most often observed. Some cohesive failure was observed in the corona-treated and corona-treated-and-primed samples, with the exception of the polyurethane, which failed to cure without treatment. It was interesting to note that many of the treated substrates provided substantial increases in tensile lap shear strength. The epoxy substrates saw limited improvement with the corona treatment but showed improvement with the addition of a primer. Adding primer to most of the treated substrates improved the lap shear strength even further. The study results confirm the belief that surface energy is a driver of bond strength. The epoxy data point does not agree with the rest of the data, and an investigation of this substrate surface should be included in future studies. The addition of the primer to this study confirms the assertion that reactive bonding sites are key to bond strength, as well.
Bonding and priming are not without material, equipment and labor costs. The inclusion of both corona treatment and priming can substantially improve the bond strength to certain substrates, but the engineer must evaluate the return on investment in this situation based on these considerations.
For more information, visit NuSil Technology at www.nusil.com.
Links
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





