Styrene Isoprene Butadiene (SIBS) Block Copolymers Improve PSA Label Adhesive Performance


Figure 1. SIBS Polymer Chemistry
SIBS polymers, like other styrene block copolymers, consist of block segments of styrene monomer and rubber monomer units. However, these polymers also contain an unsaturated elastomer, or rubber unit, consisting of isoprene and 1,3-butadiene. Figure 1 illustrates the mixed chemical mid-block of SIBS polymers. It is important to note that the unique polymerization process used to manufacture these polymers can yield both linear and radial polymer structures useful for tailoring polymers to specific performance requirements.

Preferred Label Polymer Properties
Preferred SBC label polymers have properties that provide significant adhesive formulating flexibility, such as low viscosity and acceptable strength, and can be easily tackified to yield efficiently die-cut adhesives. Polymers with high melt flow rates, a radial structure, low styrene and high diblock content are most often used to meet these requirements. The typical physical properties of Kraton MD6465BT are listed in Table 1.Characterizing the viscoelastic properties of a polymer can provide valuable insight into formulating adhesives with the desired properties. A key reason why SBCs have not been used as neat polymers in adhesive applications is that the compliance of the material (J, J = 1/G) is too low to conform to the bonding surface on a microscopic level. To develop adhesion, molecular distances between the adhesive and substrate must reach an interatomic distance of less than 1 nanometer. Dahlquist has theorized that the modulus of a material must be less than 3.3 105 Pa to adhere to a surface.
Adding low-molecular-weight materials that are compatible with the rubber mid-block phase can reduce the SBC’s modulus. Modulus reduction is accomplished by two effects: lowering the styrene content of the mixed system, and plasticizing and swelling of the rubber phase, which leads to a decrease in physical crosslink. However, simply lowering the modulus to a value less than 3.3 105 Pa by the addition of a compatible plasticizer, such as process oil, does not develop adhesion. It is found that an oiled SBC will form an intimate association with a substrate, but the bonding force is too small, and the polymer system is easily removed. To develop tack and adhesion at room temperature, the internal viscosity and resistance to peel of the elastomeric phase must be increased. Specifically, the glass-transition temperature (Tg) of the elastomeric phase must be increased from the values of the pure elastomer (isoprene = -58°C/-72°F, butadiene = -84°C/-119°F) to values between -10°C/14°F and 10°C/50°F. This is accomplished by adding tackifying resins.

Figure 2. SIBS Viscoelastic Properties
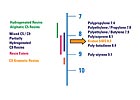
Figure 3. Polymer - Resin Solubility Parameters
Tackifying SIBS Polymers
Since SIBS polymers are styrenic block copolymers, tackifying resins and other additives have an affinity for either of the two phases with which to interact. SIBS polymers have two glass-transition temperatures - an upper (about 100°C for the polystyrene domains) and a lower (about -73°C for the isoprene-butadiene mid-block). SIBS polymers can be effectively tackified using resins from any of the major resin categories, such as aromatic modified C5 hydrocarbons, partially and fully hydrogenated hydrocarbons, rosin esters (gum, wood, and tall oil derivatives), and modified terpenes.Hildebrand solubility parameters for various polymer materials and the tackifying resin groups that are compatible with them are identified in Figure 3. As the solubility parameter for SIBS polymers’ elastomer phase, isoprene–butadiene midblock, is slightly higher (8.3) than that of the isoprene mid-block of SIS polymers (8.1), we suggest tackifying resins that contain a higher aromatic content. For example, to effectively tackify SIBS polymers using modified C5 hydrocarbon resins, those resins having an aromatic content of >10% (preferably >15%) are suggested for pressure-sensitive adhesives.

Figure 4 (top). Adhesive Viscosity Retention
Figure 5 (bottom). Low-Temperature RBT
Figure 5 (bottom). Low-Temperature RBT
Thermal Stability
There is an important difference in the mechanism of degradation between SBS and SIS polymers. SBS polymers can degrade by crosslinking in the mid-block. A formulation containing a high concentration of an SBS polymer, and held at high temperature in a mixer or holding tank for an excessive period of time, may form gel particles as degradation occurs and generally increases in viscosity. SIS polymers degrade by chain scission of the polyisoprene chain. A formulation based on an SIS polymer degraded at high temperature will show a loss in melt viscosity, but no gel will form.Because of their unique isoprene/butadiene mid-block chemistry, adhesives formulated with SIBS polymers are less subject to viscosity change during prolonged heat conditions. SIBS polymers can be mixed similar to conventional mid-block polymers, and the same level of care and good manufacturing practices should be followed. Figure 4 shows the viscosity retention in percent for hot-melt adhesive formulations containing polymer, hydrocarbon tackifying resin, naphthenic oil and antioxidant based on SIBS and SIS polymers. As demonstrated, SIBS polymers show excellent viscosity retention during heat aging.
Although many antioxidants work well and are suitable for use with SIBS polymers, Irganox® 1726 (4,6-bis [dodecylthiomethyl]-o-cresol), manufactured by Ciba Specialty Chemicals, is an exceptional antioxidant for these polymers. It is particularly effective in preventing polybutadiene gels and in retarding color generation. Antioxidant levels of 0.3-0.5% are usually sufficient for typical hot-melt applications.

Adhesive Formulations and Performance
MD6465BT will find use in PSA label adhesives ranging from high-tack permanent adhesives to lower-tack removable and specialty-type labels. Table 2 shows formulations (based on material weight percent) using MD6465BT for removable, permanent and low-temperature adhesives. The tackifying resins used are manufactured by Cray Valley.
Maintaining tack at low temperatures is critical in refrigerated and freezer label applications. PSAs using MD6465BT can be formulated to produce adhesives with superior low-temperature tack. Figure 5 shows that a 5-10°C improvement in low-temperature rolling ball tack can be expected using MD6465BT over a comparable SIS polymer. Most adhesives at lower temperatures become less tacky, which requires the use of specialty additives to achieve the necessary adhesive performance. MD6465BT, with its superior low-temperature performance, offers formulators to use fewer of these more-expensive additives, such as liquid tackifying resins.
Formulators often choose to blend SIS and SBS with SB polymers for improved economics. However, SIS polymers have limited compatibility with SBS and SB polymers in many formulations. SIBS polymers offer improved compatibility with SBS and SB polymers over conventional SIS polymers. This additional level of compatibility can help improve adhesive formulation performance. Specifically, when compared to SIS polymers, SIBS polymers:
- Accept higher levels of SBS polymer for improved economics and still maintain critical PSA performance properties.
- Produce adhesives with better heat stability and reduced gel content.
- Produce formulations with significantly improved clarity.

Conclusion
In conclusion, SIBS polymers, when formulated for PSA label applications:- Provide an excellent balance of tack, peel and shear adhesion.
- Create value and offer economical formulating latitude and compatibility with diverse ranges of formulating materials including tackifying resins, plasticizers, fillers, and additives.
- Yield a dual mechanism of chain scission and crosslinking that results in hot-melt formulations with improved viscosity stability due to their isoprene and butadiene midblock.
- Provide superior tack at lower temperatures for demanding refrigerator and freezer applications.
- Yield high-clarity adhesives when formulated with SIS, SBS and SB polymers.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!



