Introduction to Farnesene-Based Polyols for Adhesives and Sealants Formulation
A new hydrophobic polyol derived from a renewable source offers lower viscosity than hydroxyl-terminated polybutadienes (HTPBs) of the same molecular weight.

Hydroxyl-terminated polybutadiene (HTPB) resins are known to have a higher viscosity than polyether polyols of the same molecular weight. A new hydrophobic polyol derived from renewable resources, namely polyfarnesene diol, has been developed having lower viscosity than the HTPBs of the same molecular weight.
Trans-b-farnesene* was used in the present study for the anionic processes (farnesol and other oxygenated byproducts removed). Trans-b-farnesene is produced in small quantities in natural processes, but typically both a- and b-isomers are present. The supplier has modified the common yeast Saccharomyces cerevisiae to efficiently produce large amounts of trans-b- farnesene. Sugar is fermented to the target molecule in a manufacturing-ready strain that used 21 enzymatic steps in a native pathway. Only the b-isomer is active in polymerization, making this fermentation route especially attractive industrially.1
This article summarizes the preparation of polyols based on trans-b-farnesene via anionic polymerization. Polyurethanes derived from polyfarnesene diol (PFD), poly(farnesene-co-butadiene) diol, and polyol blends of polyfarnesene diol and hydroxyl-terminated polybutadiene are evaluated and compared.
Anionic Polymerization
Polymers and oligomers were produced using standard solution anionic polymerization processes. Di-functional (diol) grades were prepared with a proprietary dilithio initiator in a polar solvent. Functional grades were terminated with excess propylene oxide and neutralized with degassed distilled water, then washed repeatedly with excess water, with the solvent in the organic phase removed by steam stripping. Optimal reaction temperature was found at 30-50°C.2-4 Copolymers with butadiene were prepared by the similar process of synthesizing the homopolymers. The polymers’ molar mass was controlled by the initiator/monomer ratio.
Hydrogenation of the polymer was made on nickel catalyst in non-polar solvent. Both the non-hydrogenated and hydrogenated grades of polymer were analyzed by GPC, HPLC, DSC, and Brookfield viscometry.
Polyurethane Synthesis
Polyurethanes were prepared by a prepolymer procedure; the reaction and curing were carried out in oven held at 85°C for 5 hrs and then overnight at 60°C; each sample sheet was post-cured for one week at room temperature before physical properties were determined. Relative parameters were tested by following the procedures specified by ASTM D412, ASTM D624 Die C, and using DSC, Shore-type durometers, Brookfield viscometer, and EJA Vantage-10 tensile tester.
Results and Discussion
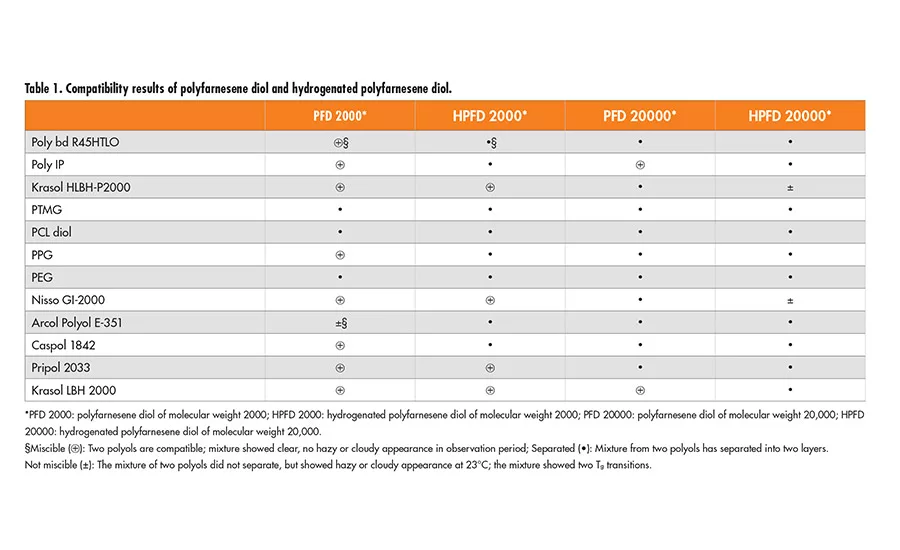
Miscibility of Polyfarnesene Diols with Other Polyols
The test was carried out by mixing either polyfarnesene diol or hydrogenated polyfarnesene diol with the select polyol in a weight ratio of 50/50. After stirring, the mixture was maintained overnight at room temperature for observation; the results are listed in Table 1 (p. 22).
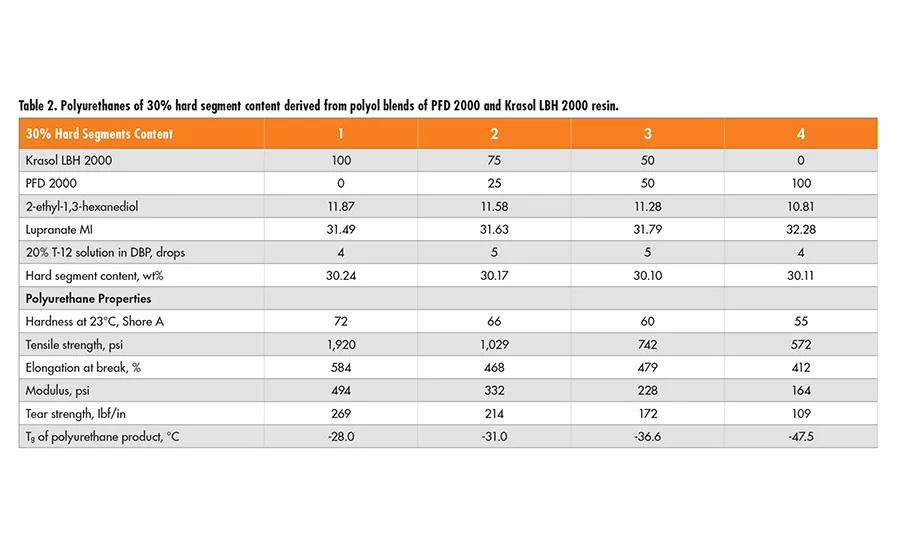
Polyurethanes Derived from Polyol Mixture Containing Diols
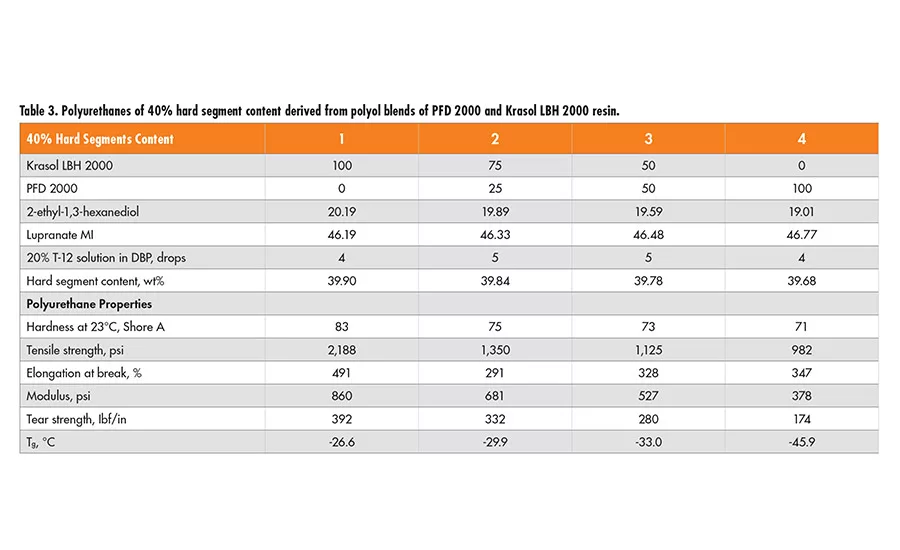
Polyfarnesene-based diol of MW 2000 (PFD2000) was used to prepare polyurethane plaques for evaluation. Two sets of formulations of 30% and 40% hard segment were designed using 2-ethyl-1,3-hexanediol (EHD) as the chain extender, and Lupranate MI (liquid MDI of 2,4’- and 4,4’-isomer mixture) as the isocyanate component. In each set, the polyol mixtures of Krasol LBH 2000 and polyfarnesene 2000 diol in a wt ratio of 100/0, 75/25 50/50 and 0/100, respectively, are used. Each formula and the mechanical properties from the resulting polyurethane are tabulated in Table 2 (p. 22) and Table 3.
In polyurethane formulas, the purpose of chain extenders is to establish a polyurethane network through hydrogen bonding, which, in turn, improves the physical properties of the final polymers. It appears that by increasing polyfarnesene diol content in the polyol mixture, the hardness, tensile strength, modulus, tear strength, and Tg of the resulting polyurethane material decrease for both 30% and 40% hard segment.
Polyurethanes Derived from Copolymer Diols Based on Different Ratios of Farnesene and Butadiene
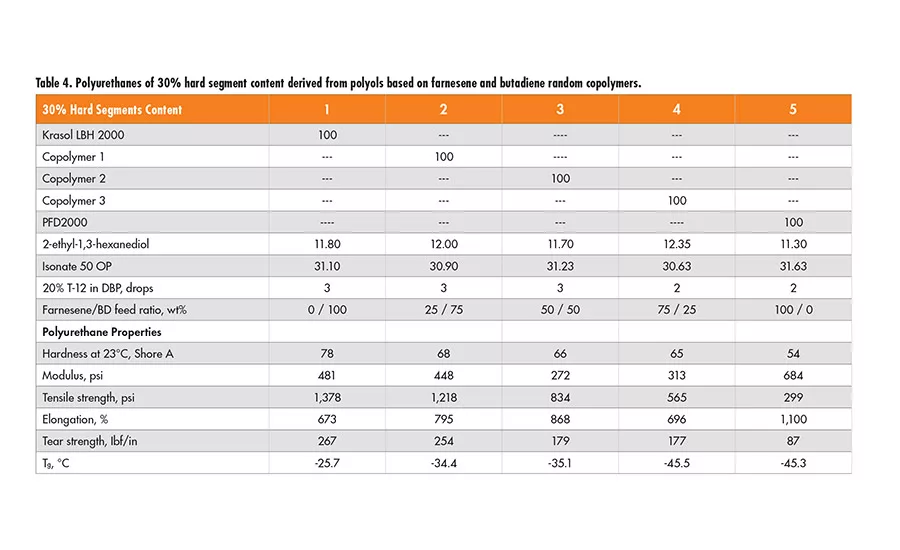
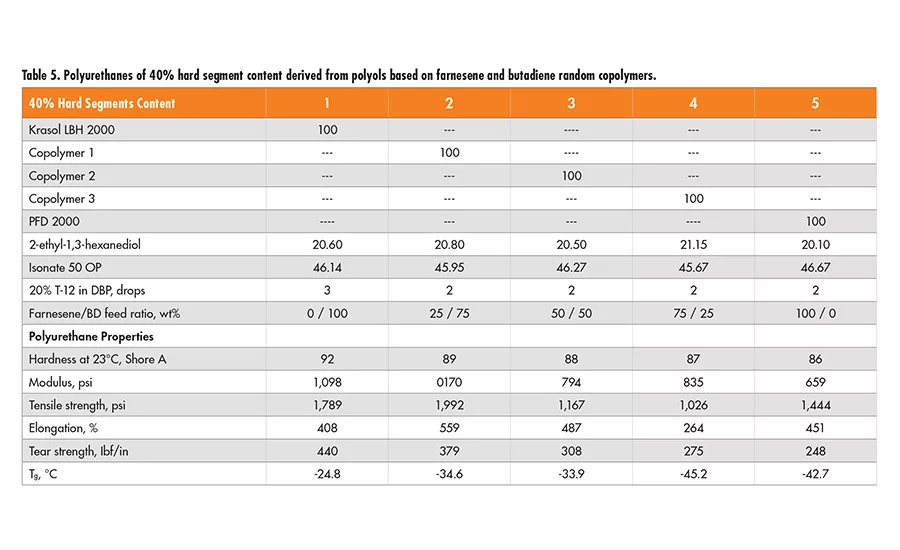
It would be interesting to compare the polyurethane properties derived from the polyols of farnesene/butadiene random copolymers with those from the polyol blends having the same monomer ratios. The polyurethanes are made with 30% and 40% hard segment content; their mechanical properties are listed in Tables 4 and 5.
Based on the properties of the polyurethanes derived from the copolymers of farnesene and butadiene, it appears that their tear strength, modulus and glass transition temperature (Tg) are nearly identical to those derived from the homopolymer polyol blends of the same farnesene-to-butadiene ratios.
Polyurethanes Derived from the Polyol Mixture Containing Polyfarnesene Diol and Poly bd Polyol
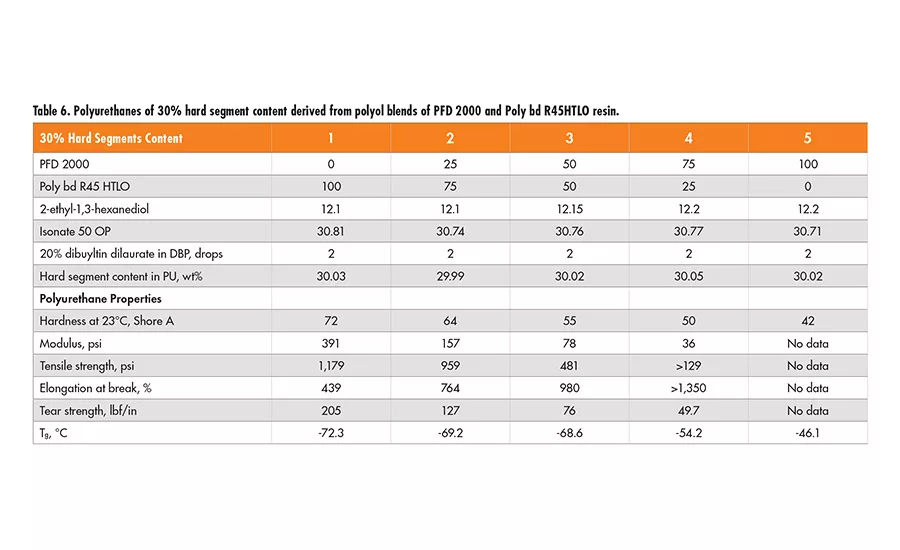
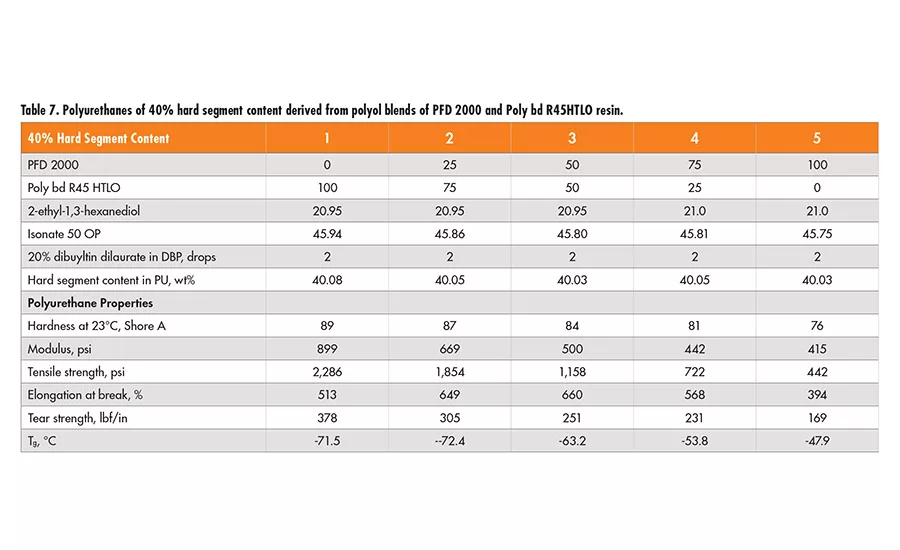
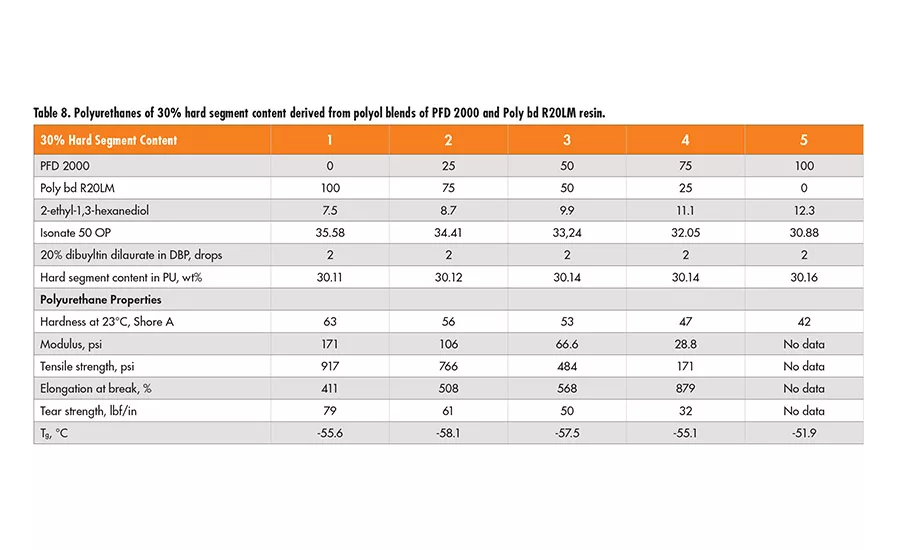
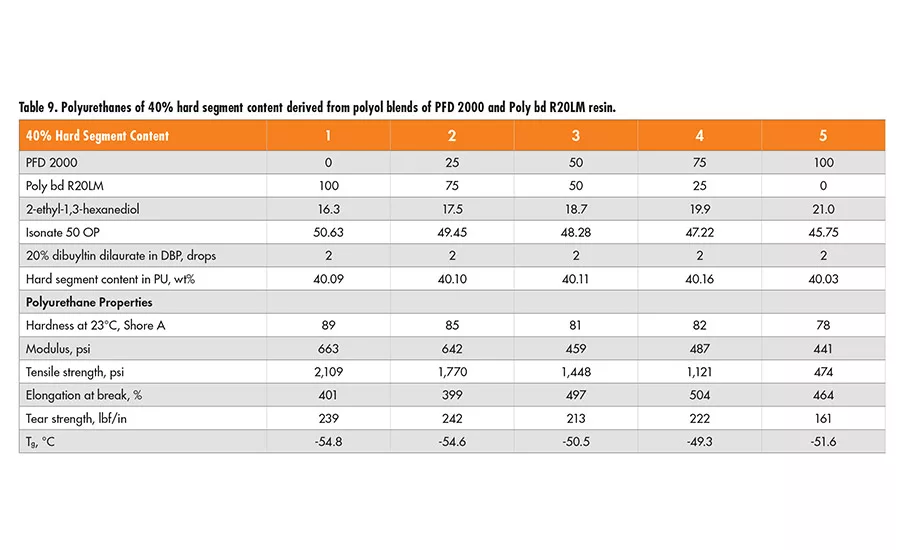
Similarly, two sets of formulations of 30% and 40% hard segment were designed using 2-ethyl-1,3-hexanediol (EHD) as the chain extender, and Lupranate MI (liquid MDI of 2,4’- and 4,4’-isomer mixture) as the isocyanate component. In each set, the polyol mixtures of Poly bd (R45HTLO or R20LM) and PFD 2000 resin in a wt ratio of 100/0, 75/25 50/50 and 0/100, respectively, were used (see Tables 6-9).
Based on the physical properties measured, it is concluded that the tensile strength of polyurethanes decreases as the PFD 2000 content increases in the polyol blend, but increases with higher hard segment content of the same polyol blend. The elongation property varies little, with all the polyol blends having 40% hard segment. However, for polyurethanes having 30% hard segment, the elongation increases with the concentration of PFD 2000 in the polyol blend and more so in the blend with Poly bd R45HTLO than Poly bd R20LM. The tear strength increases with higher hard segment content for all the polyol blends, but it decreases as PFD 2000 content increases in the polyol blends.
In addition, the polyurethanes containing Poly bd R45HTLO have better tear strength than those containing Poly bd R20LM of the same PFD/Poly bd ratios. It is of no surprise that hardness increases with hard segment content for all the polyol blends. However, the hardness decreases with increase of PFD 2000 content, regardless of the blends with Poly bd R20LM or R45HTLO resins.
Viscosity of Polyol Blends and Copolymer Polyols
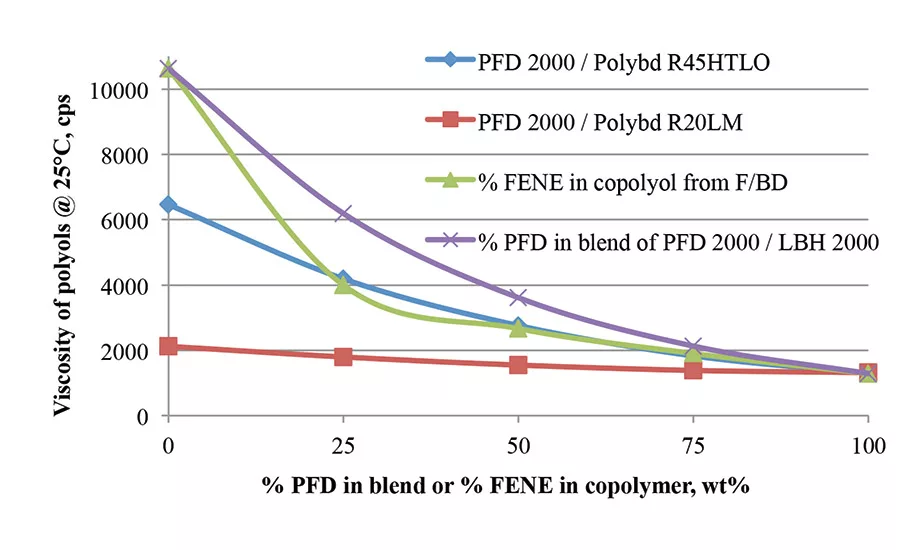
To maintain hydrophobicity of the polyurethanes derived from hydroxyl-terminated polybutadiene (HTPB) and reduce the viscosity of the HTPBs, the viscosity of polyol blends with polyfarnesene diol and the copolymers based on butadiene and farnesene in various ratios are shown in Figure 1 (p. 21). The farnesene-butadiene copolymers have lower viscosity than the polyol blends based on Krasol and polyfarnesene diols of the same monomer ratios, especially when farnesene content is below 50% in the copolymer. The viscosity decreases by adding polyfarnesene diol to Poly bd R45HTLO resin. As to the polyol blends of Poly bd R20LM and polyfarnesene diol, the viscosity reduction is not significant.
Conclusion
Farnesene was successfully polymerized using organolithium initiators in polar solvent. Quantitative functionalization was done by introducing the OH groups at the chain-ends. In comparison with hydroxyl-terminated polybutadienes of the same molecular weight, the viscosity of polyfarnesene diol is much lower. Farnesene can be copolymerized with butadiene to form copolymer polyols of various farnesene/butadiene ratios. Increasing butadiene comonomer content increases the viscosity and Tg of polymer.
Generally speaking, by increasing the ratio of polyfarnesene diol to the HTPBs, the mechanical properties of the resulting polyurethanes (such as modulus, tensile, tear strength, and hardness) are reduced. Interestingly, the properties of the polyurethanes derived from the copolymers of farnesene and butadiene appear to be nearly identical to those derived from the homopolymer polyol blends of the same farnesene-to-butadiene ratios. ASI
For more information, visit www.crayvalley.com.
References
1. D.J. McPhee, N. Moriguchi, Y. Yamana, B. Chapmon, K. Hirats, Y. Uehara, and S.K. Henning, Paper #28, Presented at the Fall 188th Technical Meeting of Rubber Division, ACS, Cleveland, Ohio, October 14-16, 2015.
2. T. Yoo, T. Trnka, S. Henning, D. McPhee, Abstracts of Papers, 251st ACS National Meeting & Exposition, San Diego, Calif., March 13-17, 2016; POLY-545 Publisher: American Chemical Society, Washington, D.C.
3. T. Yoo and S. K. Henning, Rubber Chem. Technol., 90(2), 308 (2018).
4. Henning, Steven K., 21st Annual Green Chemistry & Engineering Conference, Reston, Va., June 13-15, 2017, GC+E-50. Publisher: American Chemical Society, Washington, D.C.
*BioFene™, supplied by Amyris Inc.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!






