Strategic Solutions
PSA Wound Dressing Adhesion and Skin Type: A Delicate Balance
Consideration must be given to various skin types to ensure dressings can be securely attached and easily removed without skin damage.

Pressure-sensitive adhesives (PSAs) offer easy application and secure bonding, including adhering wound care dressings to skin. Skin is a variable substrate, however, and consideration must be given to various skin types to ensure dressings can be securely attached and easily removed without skin damage.
The global market for wound care dressings is growing. Reports show the market size for wound care dressings stands at $19 billion, growing at an average annual rate of 5-6%. Factors driving growth include population ageing, sedentary lifestyles, increases in chronic diseases (e.g., diabetes and cancer), ambulatory surgical centers making surgeries more accessible, and higher insurance reimbursements for treating chronic conditions.
PSAs and Wound Dressings
A key component of any wound dressing is the bonding layer, which attaches the dressing to the skin. The bonding layer needs to provide various functions, including adhesion, breathability, moisture vapor transmission, moisture and chemical resistance, non-irritating bond to skin, repositionability, and damage-free removal.
Though hydrogels and hydrocolloid adhesives can be used for skin bonding, these systems are thick and costly. The majority of wound dressings use PSAs that provide a cost-effective, low-profile bonding layer for dressings. PSAs remain tacky at room temperature and adhere with pressure. They can be designed to allow for breathability, moisture vapor transmission, chemical resistance, and assistance with repositionability and removal.
Three basic technologies are used in medical PSAs: synthetic rubber adhesives, acrylic adhesives, and silicone adhesives. General properties of synthetic rubber, acrylic, and silicone medical PSAs are shown in Table 1.
| Property | Synthetic Rubber PSA | Acrylic PSA |
Silicone PSA |
|---|---|---|---|
| Cost | Low | Medium | High |
| Peel Adhesive | High | Medium | Low |
| Tack | High | Medium | Low |
| Shear | High | Medium | Medium |
| Chemical resistance | Low | Medium-High | High |
| Breathability (OTR) | Low | Medium | Medium |
| MVTR | Low | Medium | Medium |
| Repositionability | No | Yes (with modification) | Yes |
| Trauma potential upon removal | High | Low-Medium | Low |
Various coating systems are used for rubber and acrylic adhesives, including hot melt, water based, and solvent based. Modifiers can be added to improve specific performance.
Performance Factors
PSAs are tested to three performance factors: peel, tack, and shear. These tests are conducted by coating adhesive to a “test strip” of flexible material. The strip is then applied to a stainless steel panel substrate.
Peel is a measurement of the force required to remove the test strip at a specified angle, applied using weight and allowed dwell time. Tack is a measurement of the instantaneous force required to remove the test strip applied using only the weight of the test strip. Shear is a test that measures the time for a test strip to remove itself from the bonding panel when under a constant weight in the same plane as the panel.
While standard PSA test methods bond test strips to rigid stainless steel panels, this is not indicative of performance on skin. Skin is variable, elastic, and textured. WebMD identifies five skin types: normal, oily, combination, dry, and sensitive. In addition, newborn skin layers are developing and sensitive to chemicals.2 Elderly skin becomes thinner and fragile, making it more susceptible to damage. Smoother skin is easier to bond than wrinkled skin.
Across all age groups, lifestyle and medical issues also add complexities. Weather and smoking can make skin wrinkled and tough. Active people may have moister skin. Chronic diseases and medications can make skin thinner, sensitive, and easily damaged. New EU Medical Device Regulations increase focus on adhesive biocompatibility and demands manufacturers perform appropriate testing on various skin types.
PSAs flow after application, depending on adhesive chemistry, improving the mechanical bond and causing adhesion to build over time. Peel adhesion tests conducted after 20 min show lower peel values than those conducted after 24 hrs. Thus, over time, adhesive flows into skin wrinkles, increasing both the bond strength and removal force needed.
Medical adhesive-related skin injuries (MARSIs) are caused by the application and removal of adhesives from skin. MARSI damage includes skin tearing and stripping, maceration, and forms of dermatitis.
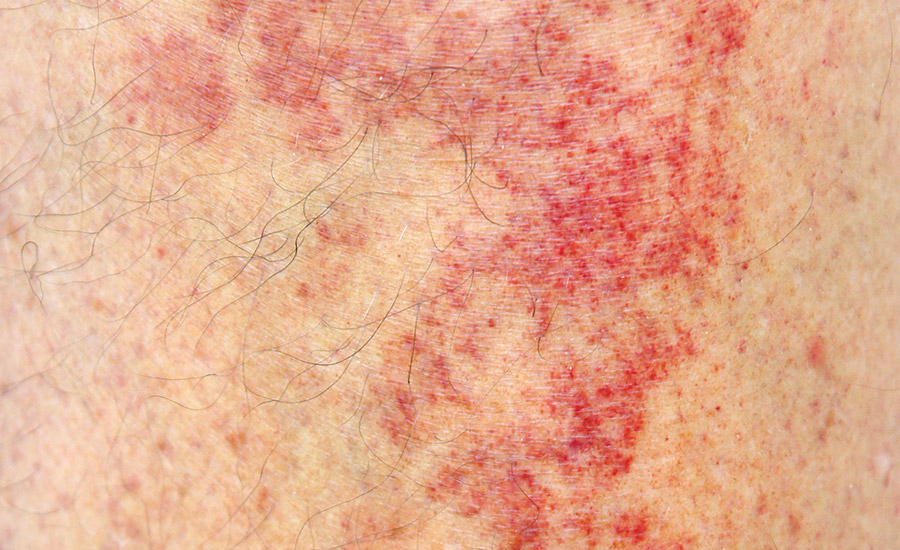
Medical adhesive-related skin injuries (MARSIs) can include skin tearing, skin stripping, maceration, and forms of dermatitis. (Image courtesy of Global Biomedical Technologies LLC.)
Two factors important to wound healing are the moisture vapor transmission rate (MVTR) and breathability, also known as oxygen transmission rate (OTR). Adhesive selection can impact these attributes.
Ongoing Development
PSA manufacturers have recognized that more offerings are needed, and development continues to yield new products for different skin types. PSA manufacturers have commercialized processes that allow coating discontinuous adhesive layers for increased MVTR and OTR, as well as reduced skin damage. Low-trauma adhesives, including silicone gel, specialized acrylics, and an “switchable on-off” adhesive system are addressing improved bonding and removal.3,4
Challenges balancing performance and cost remain, as pressure to reduce healthcare costs continues. PSA manufacturers must balance technology improvements and patient outcomes against reimbursement constraints. Research is suggested using widely available and cost-effective adhesive systems tested against refined segmentations of skin populations with the goal of providing high healing, MARSI elimination, and acceptable adhesion to various skin types.
For more information, call (513) 469-7555, email info@chemquest.com, or visit www.chemquest.com.
Note: Opening image courtesy of Artfully79 via iStock/Getty Images Plus.
References
- D.V. Varanese, “The Fundamentals of Selecting Pressure Sensitive Adhesives,” MDDI Online, January 1, 1998.
- WebMD, “What’s Your Skin Type,” March 9, 2021.
- G. Karabiyik, “Non-Silicone Adhesive for Low Trauma Skin Bonding,” MDDI Online, January 31, 2013.
- A. Wixbaux, “Advancing the Evolution of Skin Contact Adhesion Technology,” Medical Design Briefs, April 2019.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!




