A Biocide for Many Seasons
An EPA-registered sodium benzoate is approved in the U.S. for use as an in-can biocide in waterborne adhesives, coatings, inks, homecare products, raw materials, and other applications.
Proper preservation is an important contributor to sustainability, as it extends product shelf life and reduces waste associated with quickly spoiled products. An extensive study by the American Coatings Association concludes that preservation has a beneficial impact on carbon footprint.1 Preservation is also critical to protect products, manufacturers, and consumers from the risks associated with microbial contamination, such as reduced product performance, adverse health effects, and eroded consumer trust due to a potential recall.
Manufacturers of products requiring antimicrobial protection are facing an ever-diminishing list of accepted biocidal compounds. Ingredient restrictions and label requirements, in combination with non-government organization (NGO) and consumer scrutiny, have made many classical chemistries unfeasible or less desirable. Further, demand is growing for products certified to green standards, such as U.S. Environmental Protection Agency (EPA) Safer Choice.
The Green Chemistry and Commerce Council found that “green-chemistry marketed products…grew 12.6 times faster than their conventional counterparts and 5.4 times faster than the market” from 2015-2019.2 However, few chemistries become available as new alternatives because the barrier of biocide registration is quite high, requiring years, great expense, exemplary toxicology data, and proven efficacy.
With the diminishing list of available preservatives, manufacturers need new candidates with exemplary safe use profiles. Recently, sodium benzoate has become increasingly valued outside of its traditional food and beverage applications, moving into cosmetics, pharmaceuticals, home care, and industrial applications. It offers a combination of effective antimicrobial action and good water solubility, and it has an eco- and consumer-friendly profile.3 It is readily biodegradable, nature identical, listed by EPA Safer Choice as “verified to be of low concern,” and is considered non-irritating to the skin by a World Health Organization (WHO) assessment.4
Sodium benzoate is classified as a low-risk substance by the European Commission and is generally regarded as safe (GRAS) by the U.S. Food and Drug Administration (FDA).5,6 A sodium benzoate product* was registered with the EPA as a Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) biocide in the U.S. in 2020 with full state approvals in 2021.7,8 The material is approved in the U.S. for use in waterborne adhesives, coatings, inks, homecare, raw materials, and many other applications.7 (While this article will discuss the use of sodium benzoate and antimicrobial performance in adhesives, only the registered EPA FIFRA product may be used as an in-can biocide.)
The Role of pH
Organic acids have been used since ancient times through fermentation methods to preserve foods meant to sustain people well after harvest time. Organic acid preservation applies to pickling many vegetables, including cabbages, peppers, and cucumbers, and even meat in the form of fermented sausages.9,10 For food preservation, an anaerobic and low pH environment (< 4.6), in combination with salts, can eliminate or retard the growth of harmful organisms.9
The organic acids typically used to lower pH in these foods are lactic acid and acetic acid. Benzoic acid and its sodium salt, sodium benzoate, function differently, affecting more than just pH. Benzoic acid—the antimicrobial active component of the pair— is much more hydrophobic than acetic acid or lactic acid and can migrate across the cell membrane to acidify the pH inside the cell.11,12 The pH imbalance causes disruption in the cell’s ability to digest food, killing the microorganism. This ability to migrate across the microorganism’s cell membrane to disrupt cell pH balance makes sodium benzoate/benzoic acid much more powerful in terms of minimum inhibitory concentrations compared to lactic acid or acetic acid.13-15
The product pH is still critical, because sodium benzoate is the salt of benzoic acid, which is the antimicrobial active component. Thus, sodium benzoate and benzoic acid are in a pH concentration relationship. When sodium benzoate is dissolved into a water-based product, there is a reciprocal concentration of benzoic acid that varies in magnitude based on the pH of the product. The lower the pH, the higher the concentration of benzoic acid and vice versa.
This pH concentration relationship is explained by the Henderson-Hasselbalch equation.16 Based on the 4.19 pKa of benzoic acid,17 the Henderson-Hasselbalch equation can be used to model the concentration of benzoic acid as a function of pH in pure water, as shown in Figure 1.
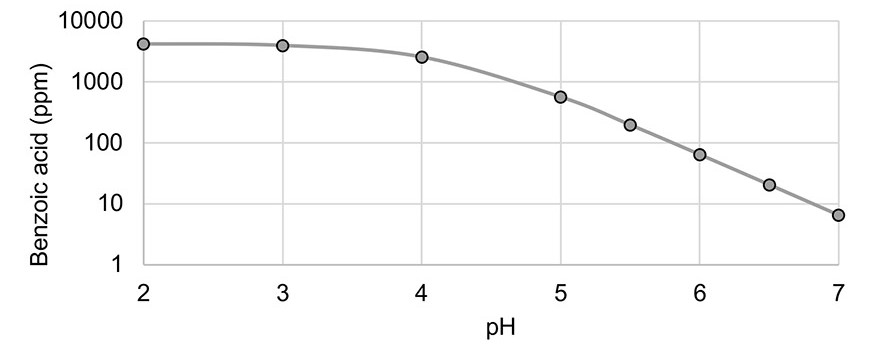
Figure 1. Benzoic acid concentration in pure water as a function of pH and 0.5% sodium benzoate.
Figure 1 is a simple model with limitations, in that:
- Products are not pure water.
- The equation ignores the effects of ionic strengths on the equilibrium.
- Anionic surfactants present in many products can beneficially increase the apparent pKa of benzoic acid, which increases the benzoic acid concentration at higher pH.18
These concepts, along with much more detailed analyses, have been published in prior literature.19 Figure 1 illustrates how strongly the concentration of benzoic acid is affected by pH. Figure 1 is a logarithmic scaling, and the concentration of benzoic acid above the pKa decreases logarithmically with pH increase.
A low, controlled product pH can be extremely beneficial for the efficient use of sodium benzoate, which will be observed later in the adhesive resin experiments. Polyvinyl acetate waterborne resins and products are often formulated or manufactured in the pH range of 3-4.5. Based on Figure 1, these materials have an ideal pH range for efficient usage of sodium benzoate due to benzoic acid concentration.
Experimental
Experiments were performed with unpreserved waterborne emulsions of polyvinyl acetate (PVAc) homopolymer resin. The EPA-registered sodium benzoate was dosed into the PVAc resin and mixed with a dispersion blade at 1,200 rpm for 15 min. The pH was measured with a Mettler Toledo FiveEasy Plus pH meter with Mettler Toledo LE438 pH probe and adjusted with 10% HCl and 10% NaOH.
PVAc resins were evaluated through challenge testing using a multiple inoculation method. The method consisted of four weekly inoculations at 106 CFU/g concentration. The following organisms were tested: Escherichia coli (bacteria), Staphylococcus aureus (bacteria), Pseudomonas aeruginosa (bacteria), Aspergillus brasiliensis (mold), and Candida albicans (yeast).
The colony forming unit (CFU) counts were assigned a logarithmic rating scale: zero if counts were < 1 CFU/g, one if counts were between 1-9 CFU/g, two if counts were between 10-99 CFU/g, three if counts were between 100-999 CFU/g, and four if counts were between > 1,000 CFU/g and too numerous to count (TNTC). To be considered passing for sufficient antimicrobial efficacy, the log reduction must show a complete kill (rating of zero) seven days following each of the four inoculations.
Results
The following figures show the results of using the EPA-registered sodium benzoate as an in-can biocide for waterborne PVAc homopolymer resins. Waterborne PVAc emulsions such as those used in this study are often stabilized with polyvinyl alcohol, which microorganisms readily digest. All of the waterborne PVAc emulsions in this study were unpreserved.
Figure 2 illustrates the microbial challenge results for the negative (unpreserved) control at pH 4. Yeast and mold remain serious challenges for this system, while the bacteria eventually adapt and thrive after multiple inoculations. The negative control shows that all five organisms will eventually thrive in the unpreserved PVAc resin.
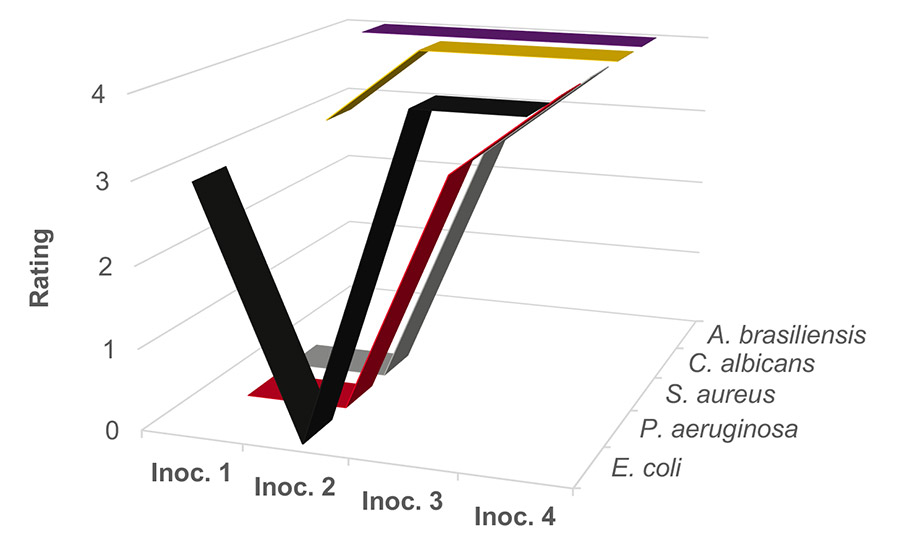
Figure 2. Unpreserved waterborne PVAc homopolymer resin at pH 4.
Figure 3 shows the results of adding 0.5% (by weight) sodium benzoate at pH 4. The bacteria is completely killed after each inoculation; however, the yeast and mold remain a challenge. The initial inoculation of mold was killed off, but subsequent inoculations resulted in the mold growing under these conditions. The yeast did not appear to be affected at this dosage and pH.
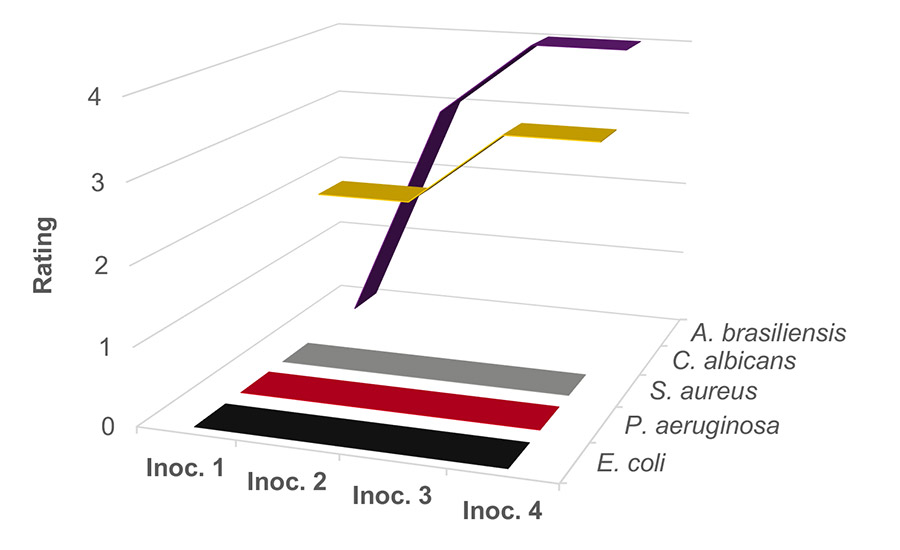
Figure 3. Waterborne PVAc homopolymer resin preserved with sodium benzoate at 0.5 wt% at pH 4.
Results at 0.75% concentration, not shown here, showed that the system was still susceptible to mold and yeast. Figure 4 shows the results of increasing the concentration of sodium benzoate to 1 wt% at pH 4. The bacteria, mold, and yeast are completely killed following each of the four inoculations. Passing a four inoculation challenge method shows that this system is well-preserved against in-can microbial contamination.
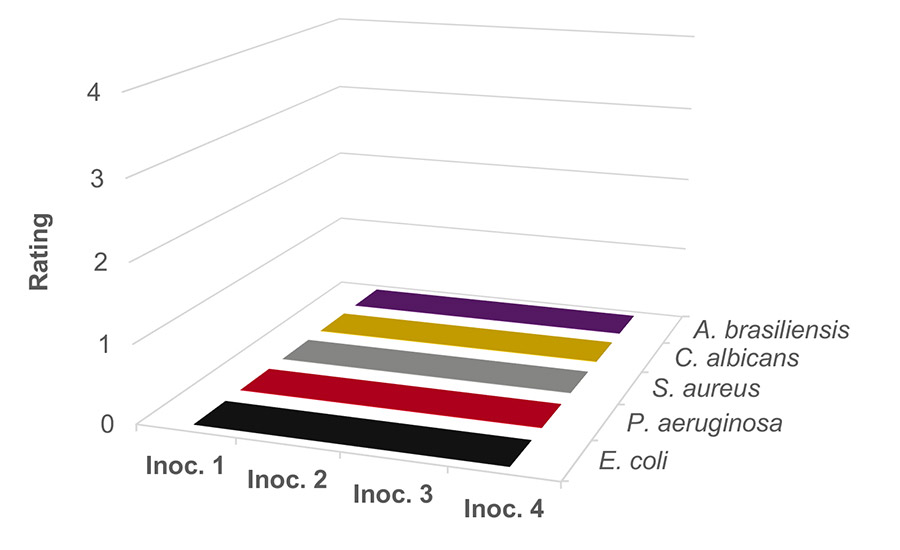
Figure 4. Waterborne PVAc homopolymer resin preserved with sodium benzoate at 1 wt% at pH 4.
While a dosage of 0.5 wt% of sodium benzoate at pH 4 did not sufficiently preserve against mold and yeast, Figure 5 shows the positive outcome of reducing the pH from 4 to 3.5 while maintaining the 0.5 wt% dosage. The bacteria, mold, and yeast are completely killed following each of the four inoculations. This result highlights the importance of the benzoic acid concentration and pH relationship shown in Figure 1. Decreasing the pH provides a more efficient usage of sodium benzoate.
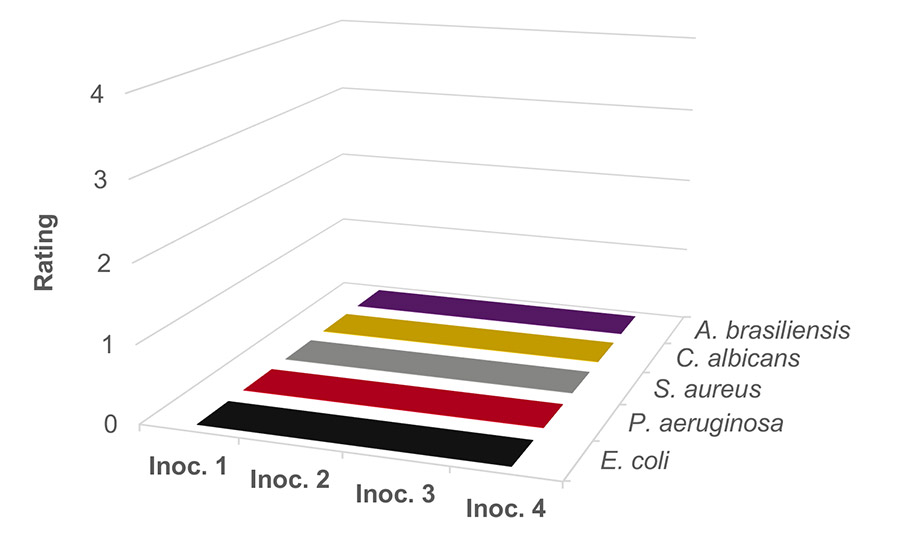
Figure 5. Waterborne PVAc homopolymer resin preserved with sodium benzoate at 0.5 wt% at pH 3.5.
Preservation Alternative
The EPA-registered sodium benzoate is approved in the U.S. for use as an in-can biocide in waterborne adhesives, coatings, inks, homecare products, raw materials, and other applications. This registration expands the palette of available chemistries to preserve these products and provides a new option with a favorable HS&E profile.
Demand for greener, non-sensitizing preservation is growing, with many classical chemistries being under scrutiny. Sodium benzoate provides a suitable alternative to meet these market needs while also achieving sufficient preservation to protect consumers, products, and brands from the hazards of microbial contamination. Lowering the pH from 4 to 3.5 can provide a significant benefit for efficient usage of sodium benzoate. Challenge testing presented herein showed that 0.5 wt% sodium benzoate can be used to produce a well-preserved waterborne PVAc resin at pH 3.5.
For more information, visit www.lanxess.com.
Note: Opening image courtesy of yacobchuk via www.gettyimages.com.
*Kalaguard® SB, a registered trademark of Emerald Kalama Chemical, LLC, a LANXESS Group company
References
- "Life-Cycle Assessment of Architectural Coatings: A World Without Preservatives. 2021," https://www.paint.org/ct-archives/%E2%80%89life-cycle-assessment-of-architectural-coatings-a-world-without-preservatives1/.
- J. Tickner, “Driving Safe and Circular Chemicals–the Business Case,” in HCPA XPAND2021 Annual Meeting, Fort Lauderdale, Fla., 2021.
- “Kalaguard® SB: Eco-friendly preservation,” https://lanxess.com/en/Products-and-Solutions/Brands/Kalaguard.
- A. Wibbertmann, J. Kielhorn, G. Koennecker, I. Mangelsorf, and C. Melber, “Benzoic Acid and Sodium Benzoate, 2000, https://www.who.int/ipcs/publications/cicad/cicad26_rev_1.pdf.
- “Opinion on Benzoic Acid and Sodium Benzoate,” Scientific Committee on Consumer Products, 2005, https://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_o_015.pdf.
- “Opinion: Benzoic acid, sodium benzoate,” Select Committee on GRAS Substances (SCOGS), 1973, http://wayback.archive-it.org/7993/20180124121624/https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/SCOGS/ucm260036.htm.
- Kalaguard® SB EPA Reg. No. 91212-1, https://www3.epa.gov/pesticides/chem_search/ppls/091212-00001-20201015.pdf.
- “Kalaguard® SB Sodium Benzoate Registered Under EPA FIFRA,” https://www.prnewswire.com/news-releases/kalaguard-sb-sodium-benzoate-registered-under-epa-fifra-301160749.html.
- K.K. Shockey and C. Shockey, “Fermented Vegetables: Creative Recipes for Fermenting 64 Vegetables & Herbs in Krauts,” Kimchis, Brined Pickles, Chutneys, Relishes & Pastes, 2014, Storey Publishing, LLC.
- W.R. Anderson, Mastering the Craft of Making Sausage, 2010, Burford Books.
- R.C. Weast, D.R. Lide, M.J. Astle, and W.H. Beyer, eds., CRC Handbook of Chemistry and Physics, 70th ed, 1989, CRC Press, Inc., Boca Raton, Fla.
- J.R. Chipley, Sodium Benzoate and Benzoic Acid, in Antimicrobials in Food, P.M. Davidson, J.N. Sofos, and A.L. Branen, Editors, 2005, CRC Press, pp. 11-48.
- W. Paulus, Microbicides for the Protection of Materials: A Handbook, 1993, Springer Netherlands.
- S. Stanojević-Nikolić, G. Dimić, L. Mojović, J. Pejin, A. Djukić-Vuković, and S. Kocić-Tanackov, “Antimicrobial Activity of Lactic Acid Against Pathogen and Spoilage Microorganisms,” Journal of Food Processing and Preservation, 2016, 40(5), pp. 990-998.
- N. Pangprasit, A. Srithanasuwan, W. Suriyasathaporn, S. Pikulkaew, J.K. Bernard, and W. Chaisri, “Antibacterial Activities of Acetic Acid against Major and Minor Pathogens Isolated from Mastitis in Dairy Cows,” Pathogens (Basel, Switzerland), 2020, 9(11), p. 961.
- L.J. Henderson, “Concerning the relationship between the strength of acids and their capacity to preserve neutrality,” Am. J. Physiol., 1908, 21, pp. 173-9.
- E.P. Serjeant and B. Dempsey, “IUPAC Chemical Data Series, No. 23: Ionization Constants of Organic Acids in Aqueous Solution,” 1979, Oxford, Pergamon.
- E. Pelizzetti and E. Pramauro, “Acid-base titrations of substituted benzoic acids in micellar systems,” Analytica Chimica Acta, 1980, 117, pp. 403-406.
- S. Foster, P. Rempala, D. Mueller, L. Dornan, A. Yarnell, and J. Blankenship, “Enhancing Antimicrobial Efficacy of Sodium Benzoate,” SOFW Journal, 10-2020, 151.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!




