Clay/Polymer Nanocomposites for Pressure-Sensitive Adhesives


Emulsion polymer suppliers, as well as many other material and product suppliers, have been practicing nanotechnology for decades. The current state-of-the-art emulsion-polymer expertise has produced a variety of high-value additives, binders and pressure-sensitive adhesives that rely on nanotechnology. Common small-sized emulsion polymers can be described as nanotechnology. Multi-phased emulsion polymers can have nanosized features and unambiguously represent nanotechnology. Figure 1 shows a number of such products developed in our research facilities. The kinetic and thermodynamic factors that control morphology development are becoming well known.4-7 Synthesis chemists have used these factors to control particle morphology, resulting in nanostructures made with hard and soft composites that have hollow cores (opaque polymer, OP),8-9 multiple lobes (ML),10 controlled shells (soluble shell polymers, SSP),11-12 high aspect ratio polymers (HARP),13 and a variety of other two- or three-phase emulsion polymers.
Polymer/Clay Nanocomposites
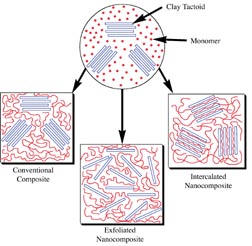
In contrast to emulsion polymer nanostructures, or to conventional composites, polymer/clay nanocomposites are an important class of the emerging new nanocomposites. These materials have demonstrated significantly enhanced properties in a number of areas, frequently delivering a superior balance of previously antagonistic properties.14 The first such application was the development of nylon/montmorillonite nanocomposites by Toyota for under-the-hood applications in automobiles.15 Such nanocomposites display an improved balance of toughness, i.e., increased modulus and tensile strength without embrittlement. Barrier properties, thermal stability and flame retardancy are also improved.14Figure 2 illustrates how polymer/clay nanocomposites differ from conventional composites.16 If a filler and polymer are brought together, such as by polymerizing a mixture of monomer and filler, an appropriately dispersed micron scale filler will usually remain approximately that size in the resulting composite (see the "conventional composite" in Figure 2). To increase stiffness or tensile strength in such composites requires on the order of 20-40% filler. With these levels of filler, other properties are often degraded, yielding undesirable outcomes such as embrittlement, lack of elongation and elimination of pressure sensitive character. While many products successfully balance these antagonistic properties in conventional composites, nanocomposites can yield property balances "outside the box." Some layered clays can be dispersed to yield nano-scale plates. For smectite clays such as montmorillonite, these layers can be as thin as 0.9 nanometers. Figure 2 illustrates two ways that polymer can access the surface of all or most of these plates in such clays. In intercalated nanocomposites, the polymer enters the gallery between the layers of clay, the clay layers maintain their registration and the increase in spacing between plates can be seen by such techniques as X-ray diffraction. In exfoliated nanocomposites, individual clay plates become dispersed in the polymer. Huge amounts of surface area are created between the polymer and the clay. For montmorillonite, surface areas in excess of 700 m2/gram have been reported. Polymer chain conformation and mobility are changed at this interface. In fact, so many of the polymer chains interact with the clay surface at levels of only 2-5% clay solids on polymer solids that bulk properties are influenced. Thus, the nanocomposite, in contrast to conventional composites, requires much lower levels of the discontinuous phase. It is more appropriate to think of the nano material as an additive than as a filler. Giannelis and coworkers3 have simulated polymer chain behavior between clay plates that demonstrates that chains close to the clay interface have lower free volume than the bulk polymer, and those away from the clay interface have higher free volume than the bulk polymer. This may begin to explain how nanocomposites can deliver an unusual balance of properties, such as increased toughness with longer elongation. Barrier properties result from the plate-like nature of the clay. Their high aspect ratio (typically 100-500 for montmorillonite) creates a "tortuous path" for materials passing through the composite.

Emulsion Polymer/Clay Nanocomposites
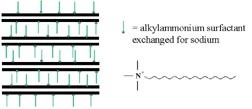
The properties of toughness (hard but flexible, improved barrier and other properties) analogous to those seen in polymer/clay nanocomposites over the last 10-14 years would be desirable in soft, film-forming latexes. However, the ability of the latex system to form a film cannot be compromised, nor, in the case of adhesives, the pressure-sensitive character of the resulting film. We report here our investigations into this area of possible use.Only limited work on emulsion polymer/clay nanocomposites has been reported.17-20 Commercially available montmorillonite clays,21 hydrophobically modified as described above, are too hydrophobic to transport through water and result in batch coagulation when introduced to an emulsion polymerization. However, sodium montmorillonite (NaMMT) disperses in water and can be introduced cleanly into an emulsion polymerization. When introduced into the early stages of an emulsion polymerization, NaMMT causes a notable increase in viscosity relative to a reaction without it. As more polymer is produced, there is an abrupt decrease in viscosity. Freeze fracture SEM22 (see Figure 4) shows that before the drop in viscosity, the structure is dominated by a "house of cards" arrangement of the NaMMT. When the viscosity drops, the latex has disrupted the NaMMT network.


Emulsion Polymer/Clay Nanocomposites as PSAs
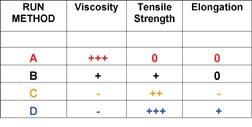
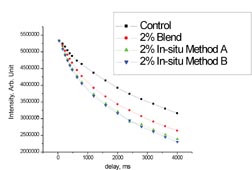
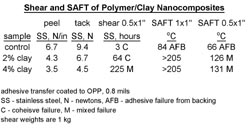
Most of the polymer/clay nanocomposites that were synthesized for pressure-sensitive adhesive testing were made using synthetic method D, as these composites demonstrated the highest interaction between the clay and the polymer. A variety of compositions and molecular weights were synthesized, which were stable and exhibited performance enhancements relative to controls made without the addition of clay. A commercially available tape adhesive was used as a control for process variations, as well as for applications testing. The clay-modified adhesives consistently demonstrated large increases in shear resistance and in shear adhesion failure temperature (SAFT) measured in a ramping oven when compared to the unmodified control. Representative data is shown in Figure 9.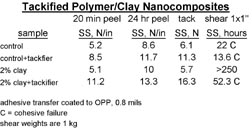
These application results demonstrate a superior balance of previously antagonistic properties for PSAs, where tack, peel and shear were all improved relative to the controls. In terms of the model for the origin of properties, 2% exfoliated clay theoretically has enough surface area to interact with about 60% of the polymer chains, confining them to a lower free volume and more rigid behavior at the clay surface. This may account for the increase in cohesive strength of the adhesive. The other 40% of the polymer chains reside in a higher free volume state than do the bulk, unmodified polymer chains. These chains have less constricted motion and exhibit more rubbery behavior than in the unmodified polymer. This allows one to produce clay-modified adhesive formulations with better tack, peel and shear properties than in non-clay controls.
Tackified, clay-modified adhesives were also run on our pilot coater on BOPP film and subsequently converted into tapes in order to assess some of the practical aspects of PSA processing. When properly formulated, the clay modification did not create any coating issues. Upon knife slitting into tape rolls, the tackified, clay-modified adhesives did not show any adhesive build up on the slitter blades, whereas the tackified controls did have build up.
Conclusion
Emulsion polymer/clay nanocomposites were successfully prepared for use as pressure-sensitive adhesives. These nanocomposites display an unusual balance of high temperature SAFT and shear properties, without overly compromising tack and peel of the adhesive. They also demonstrated an excellent tackifier response, improving tack and peel relative to the control, with better retention of shear properties. Formulated nanocomposite adhesives were successfully pilot coated without runnability issues. We also witnessed improved convertibility of these adhesive systems based on a lack of adhesive build up on the slitter blades.Acknowledgements
I would like to thank my coworkers, Debra Kline, William Finch, Chris Lester, Robert Slone and Dennis Lorah, for their synthesis and characterization efforts, as well as for their insights and technical expertise on this project. I would also like to thank Allen Arnwine for assisting me in the applications testing, and for his coating and slitting work. Kebedah Beshah, from our Spring House research site, performed the NMR studies presented here. Professor Skip Scriven and coworkers from the University of Minnesota provided the freeze fracture SEM images presented here. Finally, thanks to professor Emmanuel Giannelis of Cornell University for valuable discussions with our team.This article is based on the Carl A. Dahlquist Award paper presented at Tech XXVII in Orlando. PSTC's Tech XXVIII will take place May 4-6, 2005, in Baltimore.
For more information on polymer/clay nanocomposites, contact Rohm and Haas Co., 100 Independence Mall West, Philadelphia, PA 19106-2399; phone (215) 592-3000; fax (215) 592-3377; or visit http://www.rohmhaas.com .
References
"Polymer Nanocomposites: Synthesis, Characterization, and Modeling," ACS Symposium Series, No 804, Richard A. Vaia (Editor), Ramanan Krishnamoorti (Editor), American Chemical Society, 2002.2. Handbook of Organic-Inorganic Hybrid Materials and Nanocomposites, Vols. 1 and 2, Hari Singh Nalwa (Editor), American Scientific Publishers, March, 2003.
3. Giannelis E.P., Krishnamoorti R., Manias E. "Polymer-Silicate Nanocomposites: Model Systems for Confined Polymers and Polymer Brushes." Adv. Polym. Sci. 138: 107-147, 1999.
4. Sundberg, D.C.; Cassasa; A.; Pantazopulos, J.; Muscato, M.; Kronberg, B.; Berg, J. "Morphology Development of Polymeric Microparticles in Aqueous Dispersions I: Thermodynamic Considerations," J. Applied Polymer Science, 1990, 41, 1425.
5. Dimonie, V.; Daniels, E.; Shaffer, O.; El-Aasser, M. "Control of Particle Morphology," Emulsion Polymerization and Emulsion Polymers, Lovell, P.A.; El-Aasser, M.S., Eds.: John Wiley and Sons Ltd.: New York, 1997, 293-326.
6. Krywko, W.P.; McAuley, K.B.; Cunningham, M.F. "Mathmatical Modeling of Particle Morphology Development Induces by Radical Concentration Gradients in Seeded Styrene Homopolymerization,"Polymer Reaction Engineering, 2002, 10, 135-161.
7. Stubbs, J.M.; Karlsson, O.J.; Sundberg, E.J.; Durant, Y.G.; Johnson, J-E.; Sundburg, D.C. "Non-equilibrium Particle Morphology Development in Seeded Emulsion Polymerization 1: Penetration of Monomer and Radicals as a Function of Monomer Feed Rate During Second Stage Polymerization," Colloids and Surfaces A: Physiochemical and Engineering Aspects, 1999, 153, 255-270.
8. Kowalski, A. et. al. US Patent 4,427,836A.
9. Harren R.E. "Elements Of A Successful ResearchProject - The Development of an Opaque Polymer," J. Coating Technol. 55 (707): 79-81 1983.
10. Blankenship, R.M. et. al. US Patent 5,030,666 A.
11. Brown, A.B. et. al. US Patent 4,916,171.
12. Lorah, D.P. US Patent 4,876,313.
13. Chiou, S.-J. et. al. US Patent 5,369,163 A.
14. Ray, Suprakas Sinha; Okamoto, Masami. "Polymer/Layered Silicate Nanocomposites: A Review from Preparation to Processing," Progress in Polymer Science (2003), 28 (11), 1539-1641.
15. Kawasumi, Masaya et. al. US Patent 4810734 A.
16. Kornmann, X, Berglund, L.A., Giannelis, E.P. and Sterte, J. "Nanocomposites Based on Montmorillonite and Unsaturated Polyester," Polymer Engineering and Science 38 (8); 1351 - 1358.
17. Aguilar-Solis, Carlos; Xu, Yijin; Brittain, William J. "PVC Nanocomposites Via Emulsion and Suspension Polymerization," Abstracts of Papers, 224th ACS National Meeting, Boston, MA, August 18-22, 2002 (2002).
18. Wang, Dongyan; Zhu, Jin; Yao, Qiang; Wilkie, Charles A. "A Comparison of Various Methods for the Preparation of Polystyrene and Poly(methyl methacrylate) Clay Nanocomposites," Chemistry of Materials (2002), 14(9), 3837-3843.
19. Kim, Bo-Hyun; Jung, Jae-Hoon; Hong, Seung-Hoon; Joo, Jinsoo; Epstein, Arthur J.; Mizoguchi, Kenji; Kim, Ji W.; Choi, Hyoung J. "Nanocomposite of Polyaniline and Na+-Montmorillonite Clay," Macromolecules (2002), 35(4), 1419-1423.
20. Huang, Xinyu; Brittain, William J. "Synthesis and Characterization of PMMA Nanocomposites by Suspension and Emulsion Polymerization," Macromolecules (2001), 34(10), 3255-3260.
21. Southern Clay Products Inc. Gonzales, TX http://www.nanoclay.com/ .
22. Sutanto, Erwin; Ma, Yue; Davis, H.T.; Scriven, L.E. "Cryogenic Scanning Electron Microscopy of Early Stages of Film Formation in Drying Latex Coatings," ACS Symposium Series (2001), 790 (Film Formation in Coatings), 174-192.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





