Designed Polymer Particle Architectures for Waterborne Acrylic Pressure-Sensitive Adhesives
This paper received the 2022 Carl Dahlquist Award at the Pressure Sensitive Tape Council’s 2022 Tape Week.

Waterborne pressure-sensitive adhesives (PSAs) are the largest class of PSAs by production volume, and most waterborne PSAs comprise polymer dispersions in water. Typically, the major component of a PSA dispersion is a hydrophobic copolymer of acrylic, styrenic, or other vinyl monomers made by emulsion polymerization.
The emulsion polymerization process utilizes hydrophilic components such as water-soluble monomers, water-soluble initiators, surfactants, rheology modifiers, and salts that remain in the final PSA dispersion either associated with the polymer particles or free in the aqueous phase.1 Water-soluble monomers such as acrylic acid (AA) or 2-hydroxyethyl acrylate may copolymerize with more hydrophobic monomers so that the resulting polymer is amphiphilic, displaying hydrophilic groups on the polymer particle surface, or they may form hydrophilic polymers that remain dissolved in the aqueous phase.
Common initiators such as persulfate salts form sulfate polymer end-groups that also occupy the polymer–water interface. Surfactants that are added to PSA dispersions not only to aid polymerization but also to improve dispersion stability and coatability are present both at particle surfaces and in the aqueous phase. Salts, such as buffers or polymerization byproducts, and rheology modifiers, which are often hydrophilic polymers, are further components of the aqueous phase of a typical PSA dispersion. The aqueous phase surrounds the polymer particles, and the charged and hydrophilic components on the particles provide electrostatic and steric barriers to particle coalescence.
As this dispersion is applied and dried to create a PSA tape, label, or other article, the evaporation of water diminishes the aqueous phase and brings the particles closer together.2 Eventually, the particles deform to create a void-free film with interdiffusion of polymer chains across the particle interfaces.
Nonetheless, aqueous phase components such as surfactants, water-soluble polymer, and salts remain in the interstices between particles,3 while hydrophilic functional groups on the polymer surface can form membranes between polymer particles in the ultimate film.2 These hydrophilic domains create weak boundary layers between polymer particles that are thought to decrease adhesion, cohesion, and water resistance.4,5 Moreover, polymer particles of waterborne PSAs derived by random emulsion polymerization generally lack defined internal structures.
In contrast, studies of biological materials reveal diverse hierarchical structures that often impart superior mechanical performance.6 As shown below (a), two of these structural motifs built up in growing biomaterials are sacrificial bonds and hidden lengths.
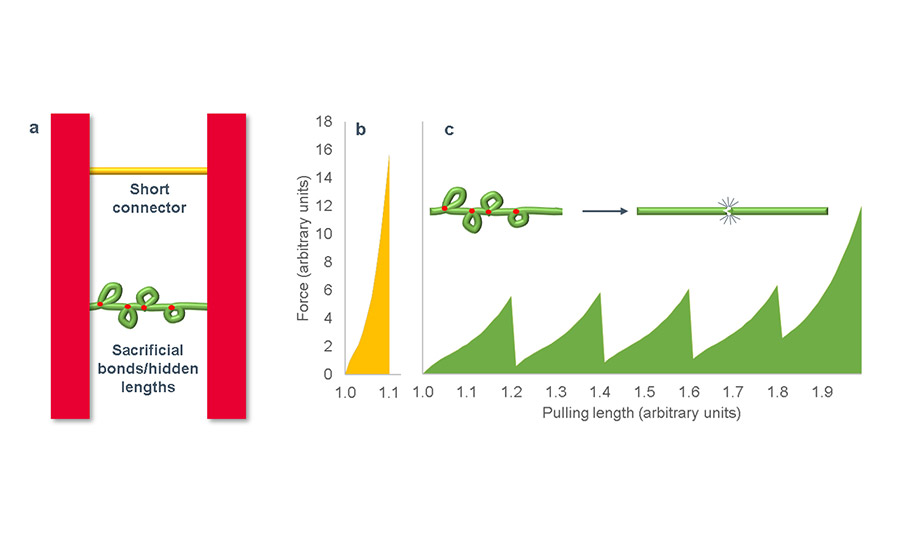
a: Diagram of bonds by a short connector (yellow) and by a connector (green) comprising sacrificial bonds (red) and hidden lengths (loops); b: Schematic of force vs. pulling length for a short connector stretched to failure; c: Schematic of force vs. pulling length as a connector comprising sacrificial bonds and hidden lengths is stretched to failure. Energy is dissipated by the scission of each of the sacrificial bonds, the unfolding of the hidden lengths, and the rupture of the entire chain. The total energy required to break the connector is much greater than that of the short connector.
Two surfaces joined by a short connector may require high force to separate them by breaking the bonds of the connector, but the total energy required to separate them is relatively low due to the short extension (b). In contrast, separation of two surfaces joined by a connector containing sacrificial bonds and hidden lengths requires multiple bond-breaking and extension steps, making the material very tough (c).
Sacrificial bonds break early in the deformation process, enabling hidden lengths in the backbone polymer to unfold prior to the ultimate rupture of the backbone. Both of these processes dissipate tremendous energy, and such sacrificial bonds/hidden lengths motifs are found in many biomaterials, including collagen, titin, tendon, and silk.6
Although the pioneering work on artificial sacrificial bond networks was performed using hydrogels,7 Ducrot et al. more recently demonstrated a tough elastomeric network employing sacrificial bonds.8 To create the sacrificial bonds, a first network comprising 6-10 wt% of the final material was polymerized from ethyl acrylate crosslinked with butanediol diacrylate (1.45-2.81 mol%) in solvent. The dried first network was then swollen to equilibrium with methyl acrylate and butanediol diacrylate (0.01 mol%), which were copolymerized in situ with UV initiation to create a double network.
Finally, the swelling and polymerization process was repeated to create a triple network in which the first network contains pre-stretched chains that can cleave at sacrificial bonds to dissipate energy as the material is deformed. This hierarchical structure incorporating sacrificial bonds dramatically increased the toughness of the triple network material to the range of that of natural rubber, which benefits from the mechanism of strain-induced crystallization.9
Inspired by these examples of hierarchical structures improving the properties of soft materials, we have explored methods to apply these concepts to waterborne acrylic PSAs made by emulsion polymerization. In this work, we attempted to build a sacrificial bond network into a PSA through multistage emulsion polymerization.
Experimental:
Preparation of PSA Dispersions by Multistage Emulsion Polymerization
The pressure-sensitive adhesive dispersions were prepared by a multistage emulsion polymerization process in a parallel automated reactor. The principal monomer composition was 97% butyl acrylate (BA), 2% styrene, and 1% acrylic acid (AA). Varying low levels of allyl methacrylate (ALMA) or divinylbenzene (DVB) crosslinkers were incorporated in some stages.
Comprising 40% of the total monomer by weight, the first stage was polymerized by gradual addition of monomer with a persulfate initiator. After the first stage was complete, the latex was cooled and 30% of the total monomer was added in one portion for the second stage of polymerization, which employed redox initiation. After the second stage was complete, the latex was cooled and 30% of the total monomer was added in one portion for the third stage of polymerization, which employed redox initiation.
Laminate Preparation from PSA Dispersions Prepared by Multistage Emulsion Polymerization
All samples were direct coated on a 2-mil polyethylene terephthalate (PET) film and closed with silicone release liner. The wet drawdowns were dried in a convection oven at 80˚C for 5 min. The samples were coated at a coat weight of 20 gsm. The samples were conditioned in a controlled temperature and humidity room (23˚C, 50% humidity) overnight prior to applications testing.
Peel Adhesion (PSTC Test Method 101)
Peel adhesion is the measure of the adhesive strength of the adhesive to the substrate. The peel adhesion is tested by applying a 1-in.-wide strip to a chosen substrate—typically stainless steel (SS) or high-density polyethylene (HDPE) panels—using a roller (4.5-lb roller moving at 24 in./min).
The adhesive is allowed to dwell on the panel for a set time (typically 20 min or 24 hr) and then peeled from the panel at a 90˚ or 180˚ angle at 12 in./min using a universal testing system. The average force is reported, along with the failure mode.
Loop Tack (PSTC Test Method 16)
The loop tack test measures the initial or instant adhesion when the adhesive comes in contact with the substrate. A 1-in. strip is cut and folded over to form a loop, exposing the adhesive side. It is then placed in between the jaws of the Instron and lowered at a rate of 12 in./min to the substrate such that a 1 x 1-in. area of the adhesive comes in contact with the substrate for 1 sec. Then the adhesive is pulled away and the peak force to pull the adhesive away from the substrate is recorded, along with the failure mode.
Shear (PSTC Test Method 107)
Shear is a measure of the cohesive strength of the adhesive. A 1 x 1-in. sample is applied to a stainless steel panel and laminated using a roller (4.5-lb roller moving at 24 in./min). The panels are mounted vertically on the shear tester at an angle of 2˚. A 1-kg weight is hung to the bottom of the sample.
The time to failure is recorded as the shear (in hours), along with the failure modes. Typically, the failure mode is cohesive, thus directly correlated with the internal strength of the adhesive.
Failure Modes
Failure modes are abbreviated as follows:
- A—Adhesive
- C—Cohesive
- AFB—Adhesive failure from backing
Part 2 of this paper, featuring Results and Discussion, will appear in the September 2022 issue of ASI.
For additional information, contact the lead author at jbbinder@dow.com or visit www.dow.com. Learn more about Tape Week at https://pstc.org.
Acknowledgements: The authors would like to thank the Dow Adhesives team for their support of this work.
References
- C.S. Chern, “Emulsion polymerization mechanisms and kinetics,” Prog. Polym. Sci., 2006, 31, 443-486.
- M.A. Winnik, “Latex film formation,” Curr. Opin. Colloid Interface Sci., 1997, 2, 192-199.
- M. Visschers, J. Laven, A.L. German, “Current understanding of the deformation of latex particles during film formation,” Prog. Org. Coatings, 1997, 30, 39-49.
- K.-H. Schumacher, J.T. Llosa, R.J. Horwitz, “Water-borne removable pressure sensitive adhesives - challenges and novel technical concepts,” PSTC TECH33, 2010.
- M. Cerra, A. Mader, “Water based pressure sensitive adhesives with enhanced water resistance for closing the gap with solvent based products,” PSTC TECH39, 2016.
- X. Zhou, B. Guo, L. Zhang, G.-H. Hu, “Progress in bio-inspired sacrificial bonds in artificial polymeric materials,” Chem. Soc. Rev., 2017, 46, 6301-6329.
- J.P. Gong, Y. Katsuyama, T. Kurokawa, Y. Osada, “Double-network hydrogels with extremely high mechanical strength,” Adv. Mater., 2003, 15, 1155-1158.
- E. Ducrot, Y. Chen, M. Bulters, R.P. Sijbesma, C. Creton, “Toughening Elastomers with Sacrificial Bonds and Watching Them Break,” Science, 2014, 344, 186-189.
- C. Creton, “50th anniversary perspective: networks and gels: soft but dynamic and tough,” Macromolecules, 2017, 50, 8297-8316.
- R. Nagelsdiek, M. Mennicken, B. Maier, H. Keul, H. Hocker, “Synthesis of Polymers Containing Cross-Linkable Groups by Atom Transfer Radical Polymerization: Poly(allyl methacrylate) and Copolymers of Allyl Methacrylate and Styrene,” Macromolecules, 2004, 37, 8923-8932.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!




