Controlling Latex Particle Size for Blush-Resistant Pressure-Sensitive Label Adhesives
Figure 1. Generalized Drawing of Latex Particles During Film Formation
Water-based latex pressure-sensitive adhesives have provided the solution for many companies looking to reduce their use of solvent-based adhesives, but they sometimes have found challenges to implementing the technology in broad-based applications. One limiting factor was its use on clear labels; latex films have a tendency to turn white or blush when exposed to water. Researchers at Ashland Inc. proposed a possible explanation for the blushing phenomena, and a practical solution.
As a latex film forms, the adhesive particles initially come together at the side exposed to air (see Figure 1). Hydrophilic material becomes trapped between those particles and naturally attracts moisture. If the film composition is semi-permeable, the hydrophilic pockets will swell when exposed to water. The swollen pockets usually have a refractive index different from that of the polymer. As the pockets swell to above 40 nm, they scatter light and the film becomes cloudy.
The degree of turbidity, or blush, depends on a number of factors. For example, if the time frame of film formation is long enough, some of hydrophilic material may follow the receding water phase. This may result in pockets with higher concentrations of hydrophilic material dispersed within the film. These pockets with more hydrophilic material will continue to swell and cause a normally clear film to blush. For example, air-dried films have been observed to have more blush than oven-dried films. In addition, the blushing of an air-dried film will not be improved by post-oven treatment. In some instances water itself can impact the blush resistance. In certain areas of the Northeast, where lead pipes are still in use, the pH of the water is lower than that of water in the Midwest. If the hydrophilic material is pH sensitive, a latex film that does not blush in the Northeast may blush in the Midwest.

Figure 2. A Comparison of the Interstice Size Between 85 and 300 nm Particles
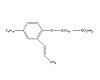
Figure 3. Hitenol BC-10

Figure 4. Blush Results for Latex Films
As companies move to water-based latex pressure-sensitive adhesives, careful attention to potential performance issues must be considered. Often, what has been seen as a problem has led to innovative solutions that further the scientific body of knowledge. In many applications, water-based adhesives provide great technology, excellent adhesion and an improved environmental profile.
Acknowledgements
The authors would like to thank Harvey Richards, Dennis Healy, Jonathan Burkhart and Kim Armstrong for their contributions to the development of this technology.For more information, contact Scott Harvey, Ashland Inc., 5200 Blazer Parkway, Dublin, OH 43017; e-mail sharvey@ashland.com.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





