Polyurethane Adhesive for Untreated Polypropylene
A new polyurethane-based adhesive has been developed to address adhesion challenges associated with bonding untreated polypropylene.


Figure 2. TG-DTA shows that decomposition temperatures tended to fall when the content of the chain extender was increased.
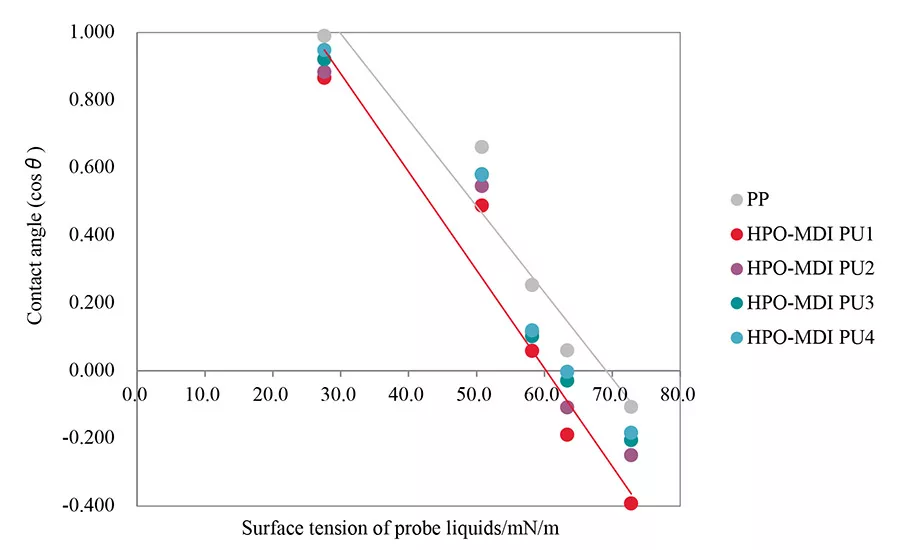
Figure 3. Zisman plot of HPO-MDI PUs and PP.





Prefer the magazine experience? Subscribe to our digital edition.
Adhesives incorporating polyurethane resin have been widely used in industry due to many desirable characteristics, such as a high adhesive property, chemical resistance, and flexibility.1 For example, these adhesives are used in the automotive industry, in both interior and exterior applications such as car body assembly, synthetic leather door panels, and center consoles.
Polypropylene (PP) is also commonly used as an important material due to its useful properties, including high chemical resistance and good heat resistance, as well as low specific gravity. In fact, PP is the most consumed engineering plastic for interior vehicle parts, finding applications in dashboards, door panels, sidewalls, roof linings, center consoles, break and accelerator pedals, and air bags. As a result, PP contributes to the reduction of car body weight and raw materials costs.2-3
However, it has been generally acknowledged that untreated PP exhibits inferior adhesion properties with other materials due to its low surface free energy.4 A previous study revealed that adhesive strength exhibits good correlation with the surface free energy of polymer substrates when an epoxy resin is used as an adhesive.5 Therefore, to improve the adhesive strength of PP substrates with other materials, the development of special pretreatments to increase the surface free energy on the PP surface have been studied. For example, acid treating, primer treating, plasma treating, and corona discharge have been reported applied in industry. In particular, the corona discharge treatment is the most widely used. It has been proposed that polar chemical groups such as carboxyl and hydroxyl groups are introduced by this treatment, and it sufficiently increases the surface free energy of the PP surface.6-7
The chemical bonding approach is well-known as the adhesion method of the PP substrate, as well as the corona discharge treatment.8 In the case of this method, the PP substrate, having reactive functional groups such as a carboxylic anhydride at the side chains, is combined with other materials by covalent bonding at interface of these materials.9-10
Although these methods are useful, they have a limitation. It can be said that it is impossible to adhere untreated PP to other materials in principle, which means that the pretreatment of the PP surface and quality control of this process is essential when using this useful material. As such, the development of an innovative adhesion method for untreated PP is quite important and meaningful. Although a number of attempts have been conducted to solve this significant technical issue, a sufficient solution has not been reported yet, mainly because most adhesives have a higher surface free energy compared with untreated PP. Theoretically, adhesives cannot adhere to PP when the adhesive has higher surface free energy than the untreated PP.10
Following the study of a variety of polyurethane compositions, a novel polyurethane adhesive has been developed that adheres well to untreated PP. It consists of hydroxyl-terminated polyolefin (HPO) and 4, 4’-diphenylmethane diisocyanate (MDI). The HPO has a low glass transition temperature, excellent hydrophobicity, and the low surface free energy derived from the polyolefin structure. To evaluate the surface free energy of synthesized HPO-MDI polyurethane resins, contact angles of various probe liquids on each polyurethane coating were measured and estimated by the Zisman plot method.11 Although this method doesn’t present a completely accurate value, it is widely used as relative comparison method. As a representative study, measurement of surface free energy of polytetrafluoroethylene by Fox and Zisman is known.12 The HPO-MDI polyurethane synthesized in this paper shows lower surface free energy than that of untreated PP.
Experimental
HPO and MDI were used as polyol and polyisocyanate, respectively. A diol compound consisting of alkyl structure was used as a chain extender. Dioctyltin dilaurate (DOTDL) was used as a catalyst for the polyurethane reaction. Cyclohexanone was used as a solvent. n-Hexadecane (> 97.0%), diiodomethane (> 99.0%), formamide (> 98.5%), glycerol (> 99.5%), and deionized water were used as a probe liquid of contact angle measurement. All materials were used as received without further purification. Untreated PP plate (JIS K 6921) and corona discharge pretreatment PET film were used as a substrate for the peel test.
HPO-MDI PU was prepared by the polyaddition of HPO and MDI in the presence of DOTDL as a catalyst in cyclohexanone at 80°C for 2 hrs under a nitrogen atmosphere. A chain extender was then added and reacted at 80°C for 6 hrs under a nitrogen atmosphere. The solid content of each sample was adjusted to 10 wt%.
The molecular weight distributions, such as Mn and Mw, were measured by gel permeation chromatography (GPC). Polystyrene was used as a standard material. The decomposition temperature was measured by thermogravimetry-differential thermal analysis (TG-DTA) under nitrogen atmosphere. The sample was heated at 10°C/min from 25-300°C.
The static contact angle of probe liquids on the surfaces of the HPO-MDI PU coatings and PP substrate were measured by contact angle meter, which allows a minimum change of 0.1° to be detected. The surface free energy of HPO-MDI PUs and PP were evaluated by the Zisman plot method using contact angle degrees.11
Untreated PP plates and corona discharge PET films were used as substrates. First, HPO-MDI PU was coated on a PP substrate using an applicator (100 µm as wet coating), and a general polyurethane-type adhesive (solid content, 10%) was coated on PET film using an applicator (100 µm as wet coating) and dried at 80°C for 5 min. These coated substrates were then stuck together as shown in Figure 1. At the end, bonded test pieces were post-treated at a predetermined temperature for 10 min.
Peel tests were carried out in accordance with a test standard (JIS K 6854-2) with a tensile tester. Peeling angle was performed at 180˚, and the peeling speed was conducted at 100 mm/min at 23°C. The test was carried out eight times on the same HPO-MDI PU sample. Maximum and minimum values were eliminated as an error data. The average value of six times was adapted as the measured value.
Results and Discussion
The HPO-MDI PUs synthesized in this paper are shown in Table 1. The content of the urethane group in the polyurethane chain was adjusted by chain extender content. The content of the urethane group increases as the content of chain extender is increased.
Average molecular weights of the HPO-MDI polyurethanes varied from 13-40 kg/mol. Molecular weight decreased when the content of the chain extender increased. This result is most likely caused by the molecular weight of the polyols. The molecular weight of the chain extender is obviously lower than HPO.
Decomposition temperatures tended to fall when the content of the chain extender was increased (see Figure 2). This tendency is probably caused by the heat stability of HPO or the heat instability of the chain extender.
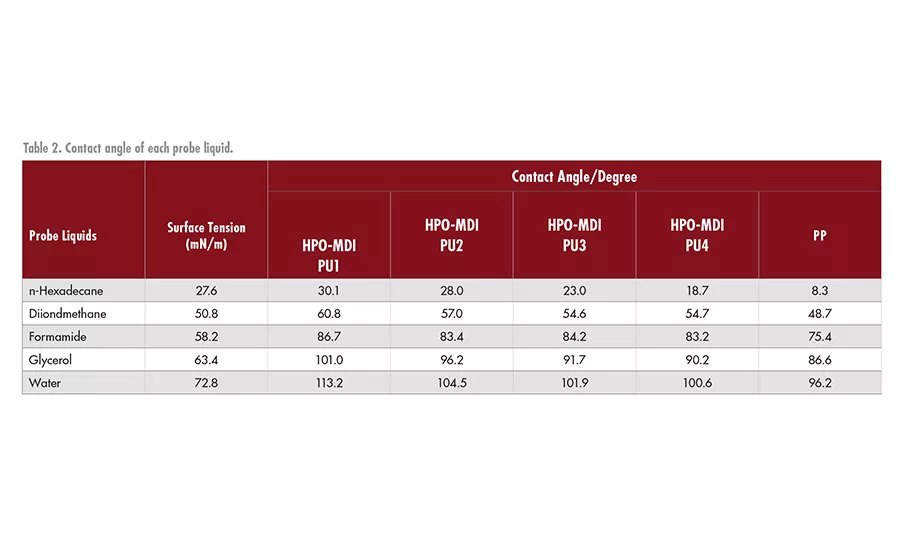
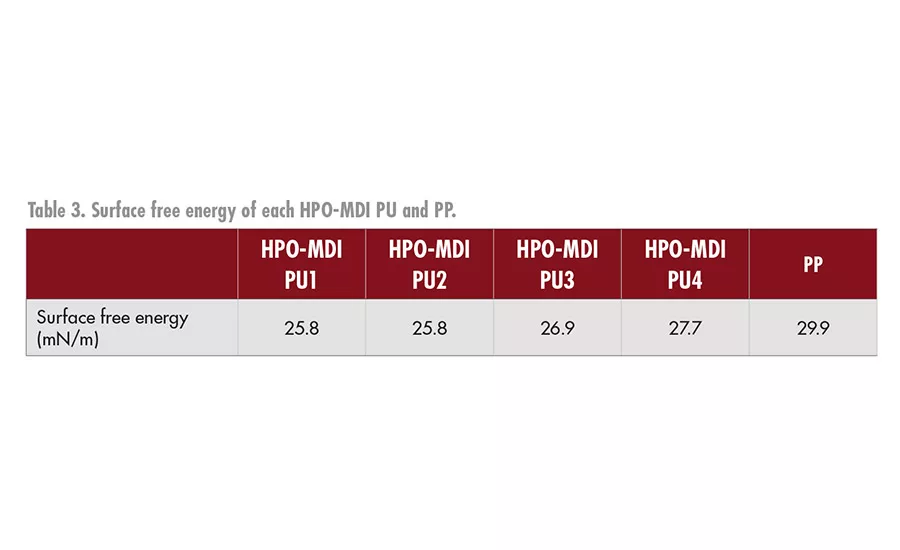
The results of the contact angle measurements are shown in Table 2. The Zisman plots of each HPO-MDI PU and PP are shown in Figure 3). The surface free energy of each sample was calculated using obtained approximate curves. The value of the horizontal axis in the case of cos =1 was taken as the surface free energy of each sample. Calculated values are shown in Table 3. This result indicates that the content of urethane groups of each HPO-MDI PU has a close relationship with surface free energy. Surface free energy increased when the content of the chain extender increased. The surface free energy of all HPO-MDI PUs showed lower values than untreated PP, which leads to the improvement of wettability of the PP surface.
The adhesive strength of each HPO-MDI PU to an untreated PP substrate was evaluated through a 180˚ peel test. The post-treat temperature of the test pieces was 80°C. A reference polyurethane-type adhesive having a higher surface free energy than PP was also evaluated. Results of the peel test are shown in Table 4. Adhesive strength decreased as the amount of chain extender increased. HPO-MDI PU1 exhibited the highest adhesive strength to PP. HPO-MDI PU3 and 4 showed the same level of adhesion as the reference. HPO-MDI PU1 showed higher adhesive strength than HPO-MDI PU2, in spite of these showing the same surface free energy. These results indicate that adhesive strength relates not only to surface free energy but also to the molecular weight of HPO-MDI PU.
In addition, it is known that the lack of surface wettability affects adhesive strength.13 This is caused by the high viscosity of neat polymer. In general, polymers have higher viscosity than pure liquids such as water and organic solvent. Therefore, polymers cannot wet the solid surface despite the surface free energy being lower than the solid surface.
In order to improve the wettability and increase the adhesive strength of HPO-MDI PU on the PP substrate, the effect of heat treatment of HPO-MDI PU1 on adhesive strength to untreated PP was evaluated. Under high temperatures, polymer viscosity decreased. Treatment temperature conditions and peel test results are shown in Table 5. There is a clear correlation between adhesive strength and treatment temperature. It was interesting to note that the adhesive strength increased obviously with elevated temperatures up to the melting point of PP. As a cause of the improvement of adhesive strength with increase of treatment temperature, the expansion of adhesion area by the improvement of the wettability is considered. Above its melting point, PP is a liquid and wets completely with HPO-MDI PU, and the adhesion is maximized. Therefore, adhesive strength is considered to be constant.
Optimized Formulation
In this paper, the influence of surface free energy on the adhesion strength of untreated PP and polyurethane adhesive is discussed by fundamental investigation, and optimized polyurethane adhesive systems are introduced. HPO-MDI polyurethanes show lower surface free energy than PP. In addition, they show excellent adhesive strength when the heat treatment temperature was 185°C. This technology provides a new solution for adhesion technology of untreated PP.
For more information, contact the lead author at yoshihiko-shiraki-ar@tosoh.co.jp or visit www.tosoh.com.
Editor’s note: This article is based on a paper presented at the 2019 Polyurethanes Technical Conference. Details regarding the conference are available at https://polyurethane.americanchemistry.com.
References
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R., “Polyurethane Types, Synthesis and Applications–A Review,” RSC Adv., 2016. 6. 114453-114482.
- Genis, A.V., “Analysis of the Global and Russian Markets of Polypropylene and of Its Main Consumption Areas,” Russian Journal of General Chemistry, 2017, 87, 2137-2150.
- Patil, A.; Patel, A.: Purohit, R., “An Overview of Polymeric Materials for Automotive Applications,” Materialstoday: Proceedings, 2017, 4, 3807-3815.
- Brewis, D.M.; Briggs, D., “Adhesion to Polyethylene and Polypropylene,” Polymer, 1981, 22, 7-16.
- Levine, M.; Ilkka, G.; Weiss, P., “Relation of the Critical Surface Tension of Polymers to Adhesion,” J. Polym. Sci. B, 1964, 2, 915-919.
- Brewis, D.M.; Mathieson, I.; Wolfensberger, M., “Treatment of Low Energy Surfaces for Adhesive Bonding,” Int. J. Adhes. Adhes., 1995, 15, 87-90.
- Földes, E.; Tóth, A.; Kálmán, E.; Fekete, E.; Tomasovszky-bobák, Á., “Surface Changes of Corona-Discharge-Treated Polyethylene Films,” J. Appl. Polym. Sci., 2000, 76, 1529-1541.
- Wake, W.C., “Theories of Adhesion and Uses of Adhesives: A Review,” Polymer, 1978, 19. 291-308.
- Duvall, J.; Sellitti, C.; Myers, C.; Hiltner, A.; Baer, E., “Interfacial Effects Produced by Crystallization of Polypropylene with Polypropylene-g-Maleic Anhydride Compatibilizers,” J. Appl. Polym. Sci., 1994, 52, 207-216.
- Chen, M.; Wan, C.; Shou, W.; Zhang, Y.; Zhang, Y.; Zhang, J., “Effects of Interfacial Adhesion on Properties of Polypropylene/Wollastonite Composites,” J. Appl. Polym. Sci., 2008, 107, 1718-1723.
- Zisman, W.A., “Relation of the Equilibrium Contact Angle to Liquid and Solid Constitution,” Adv. Chem. Ser., 1964, 43, 1-51.
- Fox, H.W.; Zisman, W.A., “The Spreading of Liquids on Low Energy Surfaces. I. Polytetrafluoroethylene,” J. Colloid Sci., 1950, 5, 514-531.
- Schonhorn, H.; Frisch, H.L.; Kwei, T.K., “Kinetics of Wetting of Surfaces by Polymer Melts,” J. Appl. Phys., 1966, 37, 4967-4973.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!







