Latex Binders, Lithium-Ion Batteries, and Electric Vehicles
Although binder adhesives are a small component of the lithium-ion batteries that power electric vehicles, they play a critical role in ensuring performance and efficiency while providing improved battery kinetics.

The electric vehicle (EV) market is witnessing exponential growth, projected to expand at a CAGR of approximately 26%.1 In turn, investment is increasing in lithium-ion (Li-ion) batteries to meet the rising market demand. Since first commercially introduced in 1991, Li-ion batteries have become an integral part of modern technology, especially for EVs. Ten years ago, the Li-ion battery could support 100 miles in an electric car. Now, it can support 200-plus miles.
Despite this progress, the increase for capacity by weight has slowed over the past three years, creating an opportunity to refine Li-ion battery components and enhance performance. Materials companies are searching for solutions to refine the Li-ion battery, increase its capacity, and enhance its charging capabilities without increasing its weight.
New innovation is required to take Li-ion batteries to the next level. Part of the solution lies with binder adhesives. Although binder adhesives are a small component of Li-ion batteries, they play a critical role in ensuring performance and efficiency while providing improved battery kinetics.
Li-Ion Batteries in the EV Market
Li-ion batteries represent a high-growth market that has seen an 18% annual growth rate from 2016-2020. Over the next five years, growth is expected to accelerate to nearly 27%, driven largely by the demand for electric vehicles. The EV market continues to gain momentum worldwide, with China, the U.S., and Germany leading the charge.
According to the International Energy Agency’s (IEA) Global EV Outlook, more than 10 million electric cars were on the roads in 2020.2 The transition from fuel to electric is not a passing fad but a lasting trend that is supported globally. Manufacturers are already planning for the future: Jaguar plans to sell only electric cars beginning in 2025; Volvo’s goal is the same for 2030, and others are following suit.
High-efficiency Li-ion batteries are the most common battery type used in EVs. These batteries have four main components: a positive electrode (cathode), a negative electrode (anode), a liquid electrolyte that helps ions move between the electrodes, and a separator to keep the electrodes from coming in direct contact with each other and to prevent short-circuits and fires. Critical for the battery operation is the flow of electron and lithium ions, which can be impaired by interactions with the battery components.
Li-ion batteries are preferred for EVs because they have higher energy density compared to lead-acid and nickel-metal alternatives. Li-ion battery cells can produce 4.2 volts, significantly higher than other technologies. This means they can deliver large amounts of electrical current for high-power applications.
Li-ion batteries are also relatively low maintenance and do not require cycling to ensure performance and maintain their battery life. Manufacturers want a battery with a long lifecycle of 10 years or more. At the end of that 10 years, the goal is to have lost only 20-25% capacity, not 100%. Although a battery capacity of 75-80% does not provide enough reach for an electric vehicle, the Li-ion battery can then be repurposed for other applications (mainly to house static storage), increasing its lifespan and sustainability.
A significant advantage of Li-ion batteries is that they have no memory effect, which occurs when batteries are recharged repeatedly after being only partially discharged. A common occurrence in nickel-cadmium and nickel-metal hybrid batteries, memory effect causes batteries to lose usable capacity and produce lower voltage.
Impact of Latex Binders
An often overlooked but critical component of Li-ion batteries is the styrene-butadiene latex binder. Composing less than 1% of the total weight of a Li-ion battery, latex binders support overall functionality and enhance performance properties. The main role of latex binders is to bind the graphite and conductivity agent powder together and onto the copper current collector in the Li-ion battery (see Figure 1).
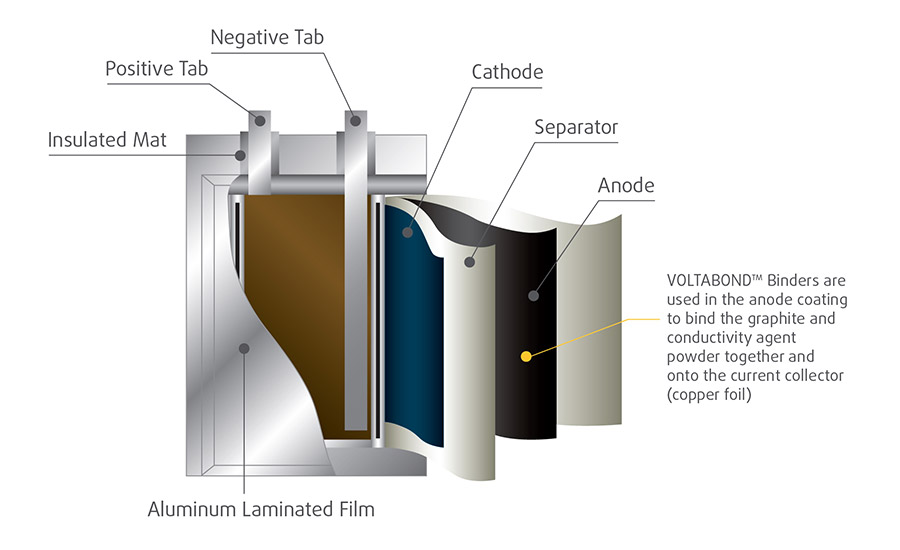
Figure 1. In lithium-ion batteries, latex binders are used to bind the graphite and conductivity agent powder together and onto the copper current collector.
Beyond high adhesion, latex binders* offer selected film formation, resistance to electrolyte swelling, elongation, and flexibility in a wide temperature range, thus enhancing the battery life cycle. Latex binders also enhance Li-ion conductivity, reduce cell impedance, and enhance the rate and low-temperature performance of the battery. They can be either solvent- or water-based, although water-based binders are often preferred as they are more cost efficient and environmentally friendly.
Multiple factors should be considered when choosing a binder, as it must be capable of performing a number of tasks. These primarily involve adhering and binding particles while also withstanding extreme temperatures and harsh conditions.
Binders must also be flexible, so they do not crack under the drying conditions or the internal extension of the graphite through loading. Brittleness can create significant problems during the manufacturing process with contact losses. During battery assembly, the binder’s elongation capacity needs to prevent ruptures and breaks through the cutting and rolling processes. During the many loading cycles, the binder also has to withstand constant interaction with the electrolyte in different temperature and pressure conditions, which can lead to fatigue breaks.
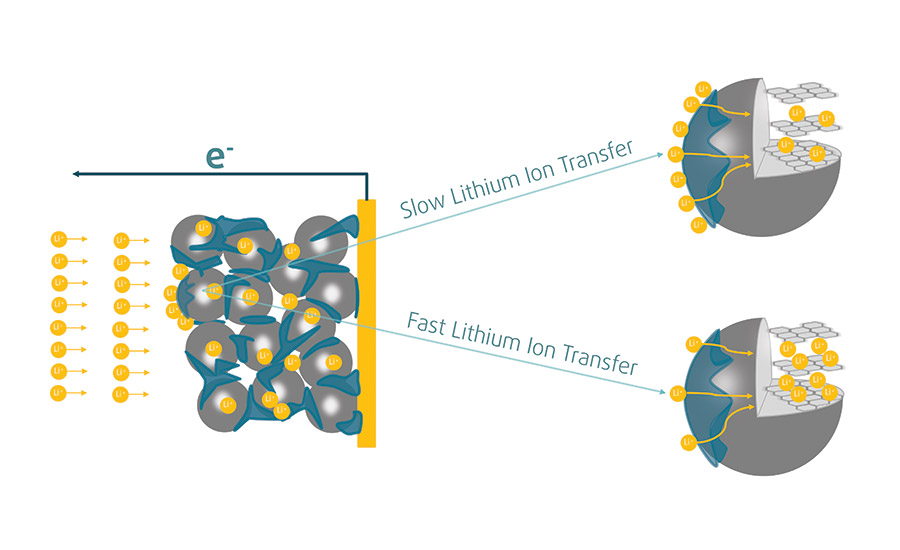
Figure 2. Binders increase the conduction rate of lithium-ions at the interface between the electrolyte and the SBR film and help improve the low-temperature performance of the battery.
Technology Improvements
Looking ahead, it is anticipated that future Li-ion batteries will be designed by partners that bring products, applications, processes, and manufacturing know-how together to build an encompassing solution with a full understanding of the interactions between these fields. The right choice of latex binders will be key to future improvements. Designed to balance electrolyte wetting and dissolving, binders possess the right adhesion and structure to deal with temperature and stress within a battery, but their full performance can only be achieved in collaboration with the other components.
Three different binding agents are currently used for each battery component (cathode, anode, and separator). A transition toward using a single binding agent for all three components would enable a cleaner system, which could benefit performance in the long term. In any component, impurities are damaging to performance over time.
The goal is to enhance the binder capacity and find an equilibrium that does not negatively interact with its environment while still adhering well to its substrates. Using one binder solution for all three battery elements and streamlining the binder chemistries may help minimize impurities, which, in turn, will increase the cleanliness of the battery, reduce capacity loss, and create a more efficient, more sustainable battery for the EV market.
In terms of improving energy consumption, researchers and manufacturers are moving quickly to develop a quality, cost-effective solution that will maintain the size and weight of the battery, along with its overall cleanliness. In addition, Li-ion research and development will likely focus on addressing many of the challenges that remain with these batteries, such as the fact that they can overheat as a result of separator failure and they age quickly due to innate chemical reactions.
The battery industry also has a critical need to create a recycling arm. The chemical processes to return lithium into its monomer state can be challenging. A collaborative approach is needed to recover the battery’s individual components and return the lithium to its original form. Future recycling solutions will need to focus on improving present materials and refining the battery chemistry and its individual components.
Future Opportunities
Li-ion batteries are the most cutting-edge battery chemistry currently available for electric vehicles, and experts widely agree no other chemistry will reach the manufacturing level to disrupt it for at least another decade.3 Li-ion batteries have changed the EV market. With many electric vehicle companies and other materials companies investing in Li-ion battery technology, the industry is unlocking new opportunities.
Listen to our Bonding with ASI podcast featuring the author!
For more information, visit www.trinseo.com.
Note: Opening image courtesy of baona via iStock / Getty Images Plus. Figures courtesy of Trinseo.
*Such as Trinseo’s VOLTABOND binders
References
1. A. Yu and M. Sumangil, “Top electric vehicle markets dominate lithium-ion battery capacity growth,” S&P Global Market Intelligence, February 16, 2021, https://www.spglobal.com/marketintelligence/en/news-insights/blog/top-electric-vehicle-markets-dominate-lithium-ion-battery-capacity-growth.
2. “Trends and developments in electric vehicle markets,” IEA Global EV Outlook 2021, https://www.iea.org/reports/global-ev-outlook-2021/trends-and-developments-in-electric-vehicle-markets.
3. A. Rathi, “How we get to the next big battery breakthrough,” Quartz, April 8, 2019, https://qz.com/1588236/how-we-get-to-the-next-big-battery-breakthrough/.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!








