Acrylic Pressure-Sensitive Adhesives
Novel, all-acrylic pressure-sensitive adhesive compositions with inherently lower surface energy display significantly improved adhesion to LSE substrates such as polyethylene and polypropylene.
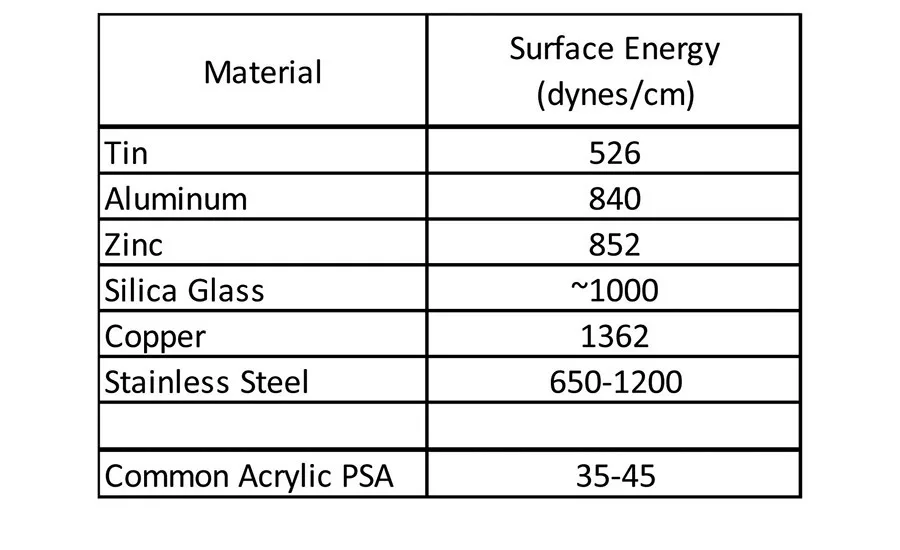
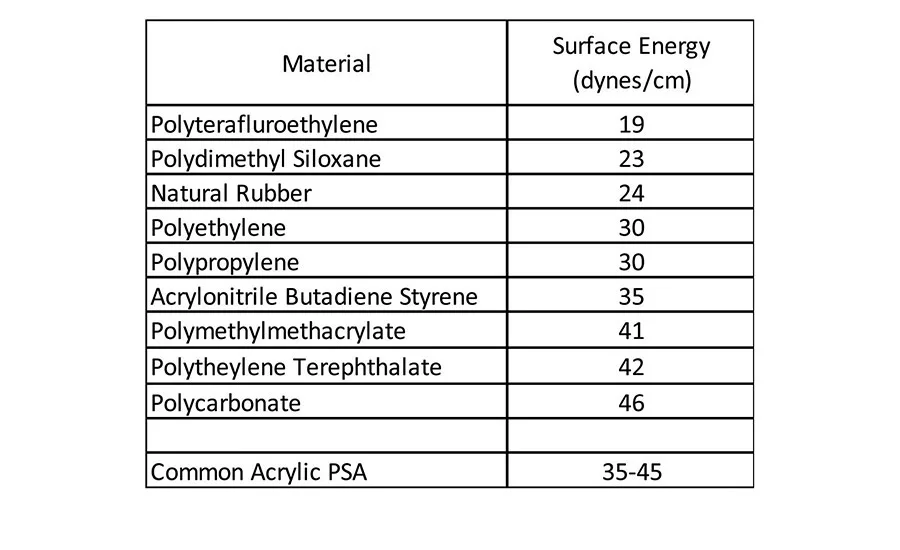
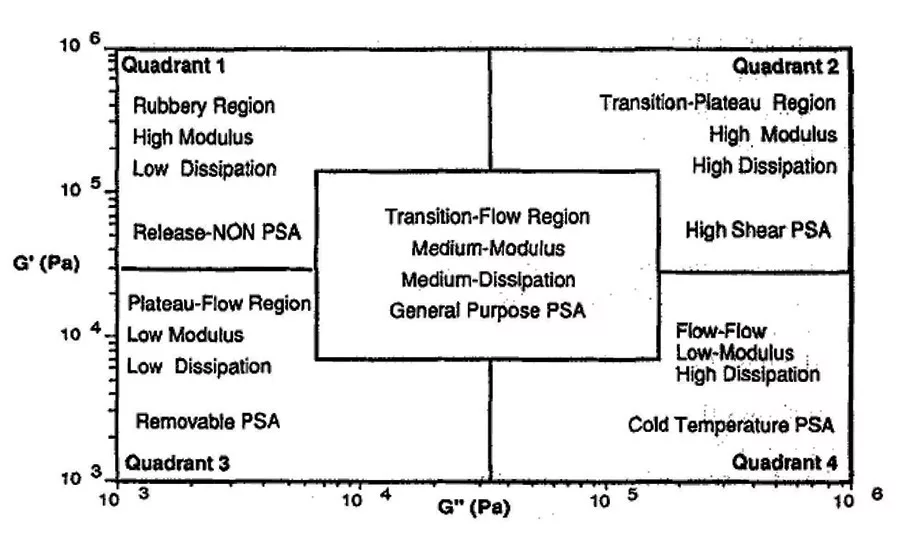
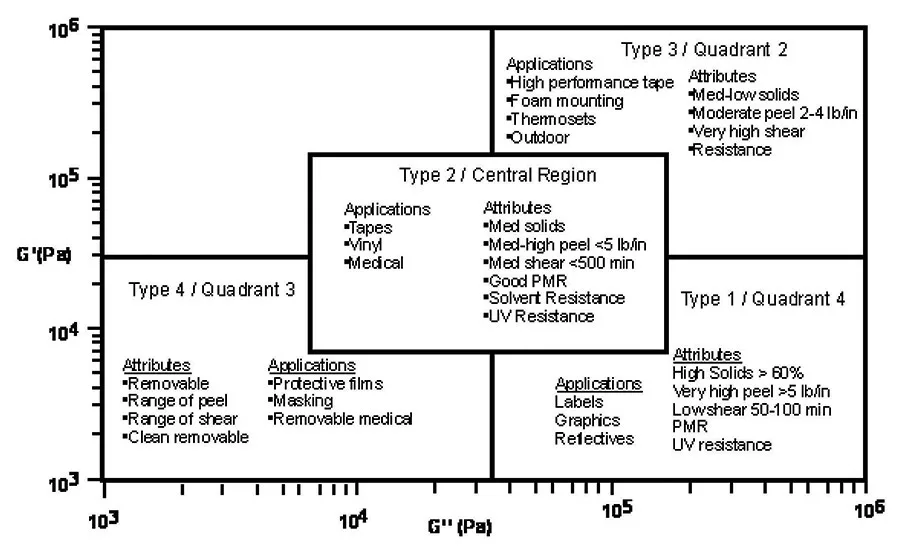
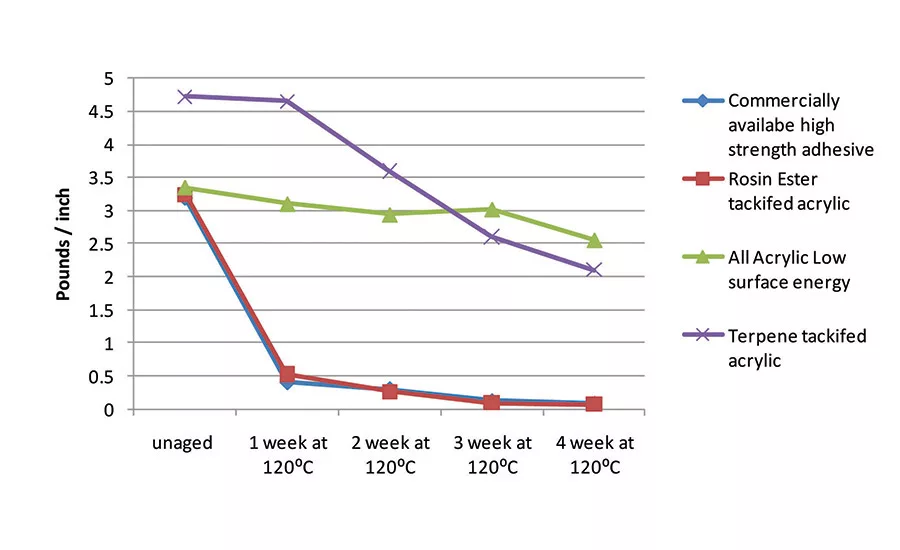



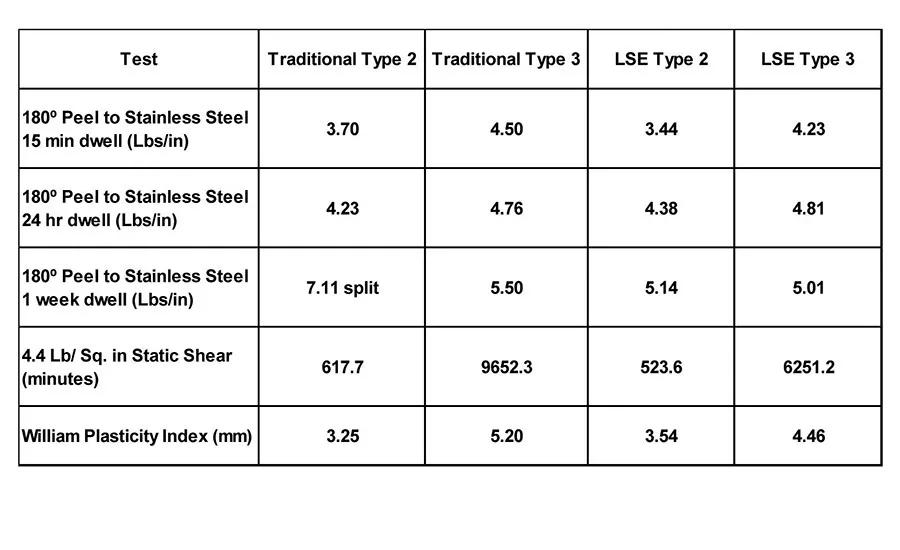
Table 6. Peel adhesion to stainless steel (4.41 lbs/in.2 static shear) and WPI of four polymers.
The prevailing trend toward the use of lighter weight and lower cost engineered plastics in automotive, construction, aerospace, electronics, and other industrial uses has created a need for pressure-sensitive materials that can bond well to these new, inherently low-surface-energy (LSE) plastics. This article discusses novel, all-acrylic compositions with inherently lower surface energy that display significantly improved adhesion to LSE substrates such as polyethylene and polypropylene. In addition, it will address evidence where these new compositions demonstrate compatibility with tackifiers historically known to be incompatible with more traditional acrylic pressure-sensitive polymers.
(Meth)Acrylic Copolymers
Pressure-sensitive polymer compositions have been used for well over 50 years. Many types of polymers can be made pressure sensitive via various formulation methods. (Meth)acrylic copolymers are one of the most widely used polymer classes for the production of pressure-sensitive adhesives (PSAs). They are relatively low cost, thermally and oxidatively stable, optically clear, and require little to no formulation to be useful pressure-sensitive materials.
Pressure-sensitive copolymers can be made from a variety of (meth)acrylic monomers. The large selection of available monomers enables a wide range of viscoelastic performance characteristics. Various chemically functional monomers provide a diverse selection of crosslinking options that can be tailored to specific applications.
(Meth)acrylic copolymers can be polymerized and used industrially in a waterborne solvent cast, melt or monomer polymer syrup. Any of these delivery formats can be selected depending on final adhesive performance requirements, the manufacturing assets available and cost requirements. Many high-performance applications require the improved coat quality, coating thickness and material properties achievable through solvent cast, or melt and syrup techniques.
Historically, (meth)acrylic pressure-sensitive adhesives (PSAs) have delivered adequate adhesion to a broad base of materials used in the industry. Ever-evolving trends in the marketplace have seen, and continue to see, the replacement of metal, glass, and wood assembly or construction materials with lower cost, lower weight plastic alternatives. This continuing trend toward lighter weight, lower cost materials has challenged the ability of traditional acrylic pressure-sensitive adhesives to adhere to these new substrates because, in most cases, these new materials have a much lower surface energy than traditional materials. Traditional acrylic pressure-sensitive adhesives adhere very well to relatively polar substrates such as steel, aluminum, tin, glass and wood. These types of materials tend to have higher free surface energy or surface tension than that of the pressure-sensitive adhesive (see Table 1).1
Given the fact that an adherend must have higher surface energy than the corresponding adherent, the common acrylic pressure-sensitive adhesive has no difficulty wetting the materials in Table 1 to form a bond surface. These types of materials have been-and are continuing to be-replaced in new material construction in various applications, including automotive assembly, building and construction, electronics, and medical devices. Many of these market areas are moving toward lighter weight and often lower cost plastic materials, but still require pressure-sensitive adhesives to bond various components. These lighter weight plastic components can be challenging to adhere to because they are generally much lower in surface energy, as seen in Table 2.1
Monomer Selection
As mentioned previously, a variety of (meth)acrylic monomers is available for use in the design of an acrylic pressure-sensitive adhesive. These materials can be fairly polar or non-polar, depending on the length and chemical nature of the ester side chain. The polarity of a monomer can be expressed as solubility parameter2 (cal/cm3),1,2 and selecting a monomer with lower solubility parameters results in a final adhesive that can wet lower surface energy materials.
Some common acrylic base monomers, functional monomers, and polar/non-polar glass-transition temperature (Tg) modifying monomers, along with their corresponding glass-transition temperatures and solubility parameters, can be found in Table 3.3 Designing an acrylic pressure-sensitive adhesive with monomers that have lower solubility parameters will inherently enable the pressure-sensitive adhesive to wet a wider variety of materials, which can include various plastic substrates.
In Viscoelastic Windows of Pressure Sensitive Adhesives,4 E.P. Chang details a quadrant approach of classifying polymeric materials by loss and storage modulus measurements. This method uses mechanical analysis to predict the ability of a material to flow. It does not predict wetting because it does not include surface energy effects. However, using these techniques, general application quadrants can be identified by material flow properties, as seen in Figure 1.
From these quadrants, pressure-sensitive adhesives can be classified by their flow characteristic into types, which often translate into applicable market applications. As seen in Figure 2, quadrant 4, high G’’ low G’ materials, or Type 1 pressure-sensitive adhesives, generally are high-peel and low-shear materials that are usually high solids. These materials tend to be water based and are used in markets such as labels and graphics.
Type 2, the central region of the viscoelastic window, includes moderate- to high-peel and moderate-shear materials. Type 2 pressure-sensitive adhesives are used in some high-performance label, vinyl graphic and medical applications. Quadrant 2 from the viscoelastic windows, or Type 3 adhesive, includes moderate-peel and high-shear materials. Type 3 pressure-sensitive adhesives can be used for high-performance tape applications. Quadrant 3, or Type 4, materials include a range of removable or temporary adhesives used for protective films and medical applications.
A tool used in designing an acrylic pressure-sensitive adhesive of desired viscoelastic properties is to modify the glass transition of the material. Traditionally, this is done by incorporating high Tg modifying, or chemically functional monomers, as shown previously in Table 3. However, incorporating significant amounts of these monomers into an acrylic-base polymer can raise the solubility parameter of the material such that it can no longer wet low-surface-energy substrates, thereby preventing an intimate bond.
One traditional method of making an acrylic pressure-sensitive adhesive chemically functional, so it can be chemically crosslinked, is through the incorporation of acrylic acid. Acrylic acid has a low molar equivalency weight and relatively high Tg, and can stiffen the acrylic-base polymer because the acid functionality is very close to the main polymer chain. High molar equivalency weight acid functional acrylic monomer exists in the market. These materials tend to be lower Tg and do not stiffen the main acrylic base polymer as much as acrylic acid because the acid group is further removed from the backbone.
Because the acid functionality on these materials is separated from the main chain by some chemical spacing group, the acid functionality is better able to take part in hydrogen bonding with a substrate. Higher molar equivalency acid functional materials also result in an overall lower solubility parameter polymer because there are less acid groups than an acrylic acid-containing system. These functional monomers are often the adducts of alcohol or amine functional (meth)acrylics and an anhydride, or ring-opened cyclic compounds, such as caprolactam.
Testing
Unless otherwise noted, the test methods shown in Table 4 were used for evaluating the adhesive properties of the acrylic polymers. Two acrylic-base polymers have been developed using low solubility parameter Tg-modifying and a high equivalency weight acid functional monomer. The new Type 2 and Type 3 polymers have a Tg of -45°C and -35°C, respectively, as shown in Table 5.
The polymers were cast from solvent and crosslinked with aluminum acetyl acetonate. They were then compared to traditional, commercially available Type 2 and Type 3 polymers that contain short-chain polar Tg-modifying monomers, and acrylic acid. All pressure-sensitive adhesive testing was performed at a 50 g/m2 coat weight, using a polyethylene terephthalate (PET) face stock. Test methods conform with PSTC 101.
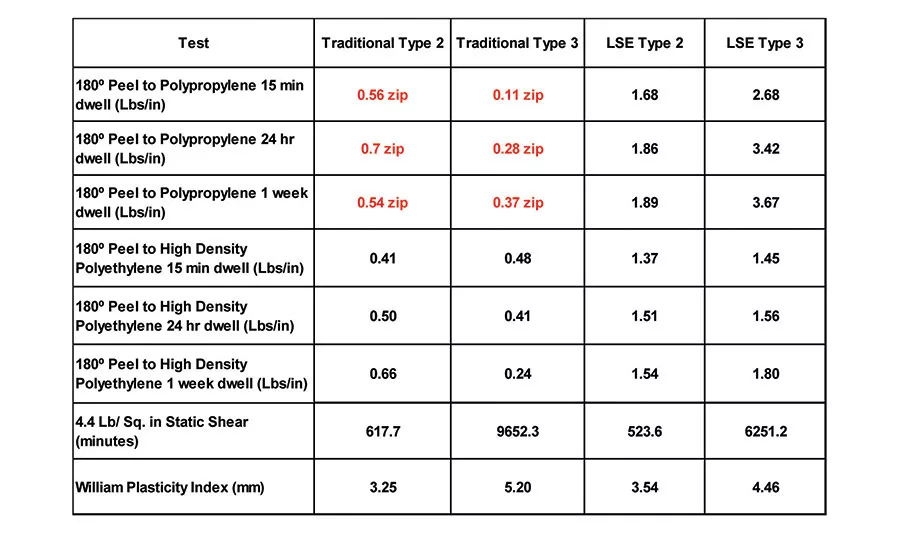
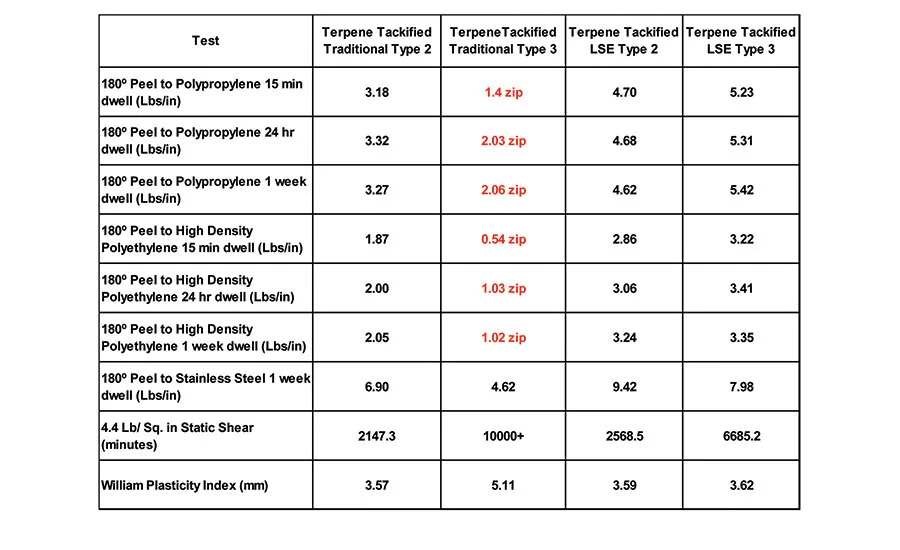
Table 6 displays the 180° peel adhesion to stainless steel (4.4 lbs/in.2 static shear) and the WPI of the traditional Type 2 and Type 3 polymers, compared to the LSE Type 2 and Type 3 polymers. The properties of the new polymers are comparable to the traditional acrylic PSAs when measured on stainless steel. However, when the adhesion to common olefin substrates, like polypropylene and high-density polyethylene, are measured, significant differences are evident (see Table 7). Both the traditional Type 2 and Type 3 polymers exhibit low peel values and severe zipping or slip stick on polypropylene, and smooth but low adhesion values on high- density polyethylene compared to the new LSE compositions.
Tackification
The addition of a tackifying resin is an often-used method of improving adhesion to low-surface energy substrates. Tackifying resins are used to raise the Tg, lower the modulus, and often lower the solubility parameter of an adhesive formulation. Tackifying resins can be classified into three groups: hydrocarbon resins are based on petroleum feed stocks and are synthetically polymerized; rosin- and terpene-based resins are derived from natural feed stocks and then chemically modified; and rosin ester tackifiers (one of the most common tackifiers used in acrylic pressure-sensitive adhesives) are the product of esterification of crude rosin acid with glycerol or other common multi-functional alcohol.
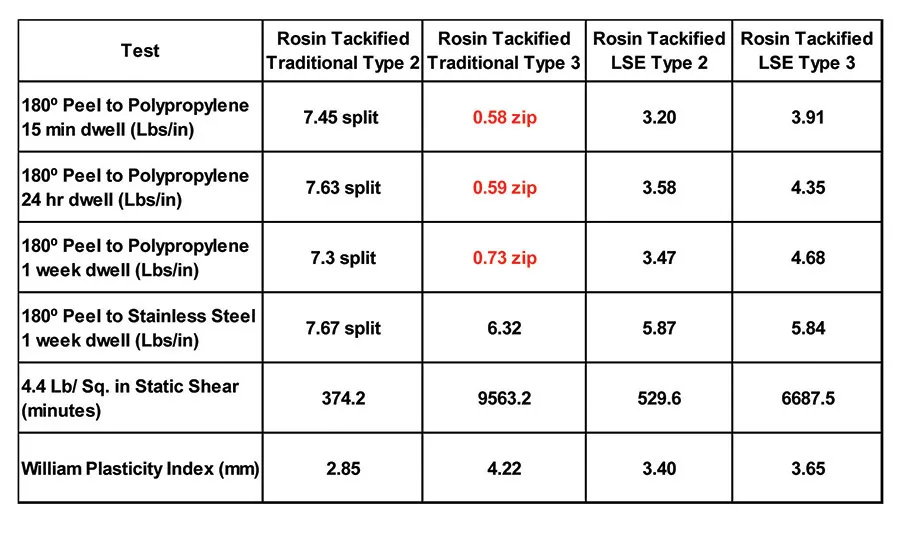
Table 8 displays the adhesion performance, on both polypropylene and stainless steel, of a 20 parts-per-hundred (PPH) addition of an 85°C softening-point rosin ester to the base polymers mentioned above. The rosin ester tackifier enabled the traditional Type 2 polymer to adhere well to polypropylene, although the traditional Type 3 polymer still exhibited zipping and low adhesion with the rosin ester addition. Both LSE polymers exhibited the expected increase in peel associated with the tackifier addition while maintaining high shear performance.
Terpene resins are produced by oligomerization of pinene and limonene to produce non-polar tackifiers with a low molecular weight and a high softening point. Terpene resins are not commonly used in acrylic pressure-sensitive adhesives because, traditionally, they have very limited solubility in acrylics containing polar monomers. However, when low-solubility acrylic monomers are used to design an acrylic pressure-sensitive adhesive, like those of the LSE Type 2 and Type 3 mentioned previously, these non-polar tackifers become useful. Table 9 displays the adhesive performance of a 105°C softening-point polyterpene resin addition at a 20 PPH loading level to the four polymers above.
The terpene addition to the LSE-type polymers results in a good, broad-based adhesive to low- and high-surface energy substrates, as well as excellent shear. However, the terpene resin results in a slight improvement in the case of the traditional Type 2, and zipping failures when added to the traditional Type 3.
In addition to enhanced adhesion to low-surface-energy substrates, the LSE compositions and the terpene-tackified materials offer increased thermal stability over traditional rosin ester-containing acrylic pressure-sensitive adhesives. Thermal aging of several adhesive constructions was performed at 120°C on propylene test panels. Adhesion was measured prior to thermal exposure and every week thereafter for four weeks.
Figure 3 displays the peel adhesion measured at each interval for a commercially available high-strength adhesive; a rosin ester-containing acrylic; an all-acrylic, low-solubility parameter composition; and a terpene-tackified low-solubility parameter acrylic. The rosin ester-containing acrylic and the commercially available high-strength adhesive experienced a dramatic decrease in adhesion upon thermal exposure. However, the all-acrylic composition retained virtually all its adhesion through four weeks of aging.
The terpene-containing sample lost about 50% of its peel force over the four-week aging period, which is still superior to that of the control. This decrease in peel exhibited by the tackified materials is likely caused by residual unsaturation being oxidized in the tackifiers. The rosin ester used in this study is hydrogenated, but hydrogenation is never driven to 100% conversion. The terpene materials are inherently more saturated than the rosin-based tackifiers, but still contain some unsaturations. However, the terpene-tackified material offers a thermal stability improvement over rosin ester-containing adhesives.
Inter-Penetrating Networks
Polyacrylate polyether inter-penetrating networks (IPNs) have been previously disclosed.5 These materials combine the elastomeric, high modulus and flexibility of a sealant with the wetting and viscoelastic characteristic of a pressure-sensitive adhesive to produce a hybrid IPN high-performance PSA. Silane-functional telechelic polypropylene oxide is a class of reactive oligomer that can be used to make the IPN materials.
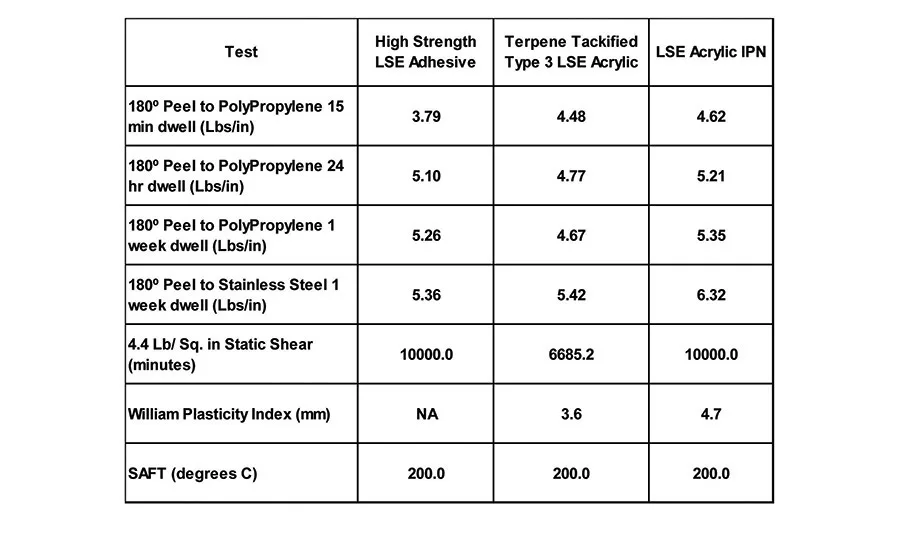
The combination of the polypropylene oxide oligomer, which has a very low solubility parameter compared with the low solubility parameter of acrylic polymers previously discussed, allows for the production of high-performance adhesives that adhere very well to olefin substrates. Table 10 details the adhesive performance of an IPN-made using the Type 3 LSE acrylic discussed above, a propylene oxide oligmer and a terpene resin-in comparison to the terpene-tackified Type 3 LSE polymer and a commercially available high-strength LSE adhesive.
The IPN material exhibits enhanced peel and shear properties over the terpene-tackified base polymer alone. It also displays significantly higher WPI, indicating that the IPN material will slit and convert much easier. The enhancement in peel, shear and WPI suggests some synergistic effects of the polyacrylate polyether IPN system.
Conclusion
The expanding use of lightweight LSE plastic materials in many common applications requiring pressure-sensitive adhesives has created the need for materials that can bond well to these new substrates. Acrylic pressure-sensitive adhesives exhibiting enhanced adhesion to these LSE plastics have been demonstrated.
Common formulation additives, such as tackifiers, can be used. In addition, low solubility parameter tackifying resins-such as polyterpene resins-have shown applicability with these new acrylic pressure-sensitive adhesives. These all-acrylic and terpene-tackified pressure-sensitive materials also offer enhanced thermal stability over traditional rosin-containing adhesives. Finally, IPNs made with these low-solubility parameter acrylics enable the production of a high-performance hybrid pressure-sensitive adhesive.
For additional information, contact Avery Dennison Corp. at 250 Chester St. #5M, Painesville, OH 44077; phone (866) 462-8379; fax (440) 358-3298; email psa.tape@averydennison.com; or visit www.averydennison.com.
Editor’s note: This article is based on a paper presented at the Pressure Sensitive Tape Council's TECH 34 conference.
References
- Petrie, E., Handbook of Adhesive and Sealants, McGraw-Hill, New York, 2000.
- Fedors, R., “A Method for Estimating Both Solubility Parameters and Molar Volumes of Liquids,” Polymer Engineering, February 1974, Vol. 14, No 2.
- Brandrup, J., Immergut, E. H., and Grulke, E. A., “Glass Transition Temperatures of Polymers,” Polymer Handbook, Wiley, New Jersey, 1999, Vol. 1.
- Chang, E. P., “Viscoelastic Properties of Pressure-Sensitive Adhesives,” The Journal of Adhesion, 1997, Vol. 60, pp. 233-248.
- Zajaczkowski, M., “Acrylate-Polyether Based Pressure Sensitive Adhesives,” Proceedings of the Pressure Sensitive Tape Council Tech 30 Global Conference VI, Orlando, FL, May 16-18, 2007, Pressure Sensitive Tape Council.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





